SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 20-F/A
(Amendment No. 1)
☐ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
Or
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2018
Or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Or
☐ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission File No. 001-36203
Can-Fite BioPharma Ltd.
(Exact name of Registrant as specified in its charter)
Can-Fite BioPharma Ltd., an Israeli Limited Company
(Translation of the Registrant’s name into English)
Israel
(Jurisdiction of incorporation)
10 Bareket Street,
Kiryat Matalon,
P.O. Box 7537,
Petah-Tikva
4951778, Israel
(Address of principal executive offices)
Motti Farbstein
Chief Operating and Financial Officer
Tel: +972 (3) 924-1114
Fax: +972 (3) 924-9378
motti@canfite.co.il
10 Bareket Street,
Kiryat Matalon,
P.O. Box 7537,
Petah-Tikva
4951778, Israel
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
American Depositary Shares, each representing 2 Ordinary Shares, par value NIS 0.25 per share
(Title of Class)
Ordinary Shares, par value NIS 0.25 per share*
(Title of Class)
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
* Not for trading, but only in connection with the registration of the American Depositary Shares.
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report (December 31, 2018): 40,399,290 ordinary shares are outstanding.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such a shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | Non-accelerated filer | ☒ |
| Emerging growth company | ☒ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP ☐ | International Financial Reporting Standards | Other ☐ |
| as issued by the International Accounting Standards Board ☒ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the Registrant has elected to follow: Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
Explanatory Note
This Amendment No. 1 to the Annual Report on Form 20-F of Can-Fite BioPharma Ltd. (the “Company”) for the year ended December 31, 2018 filed with the Securities and Exchange Commission on March 29, 2019 (the “Original Filing”) is being filed to correct the number of ordinary shares issuable upon exercise of outstanding warrants on page 24 in “Item 3.D. Risk Factors”. This Amendment No. 1 also includes an updated signature page, the certifications of the Company’s Principal Executive Officer and Principal Financial Officer in Exhibits 12 and 13, and the consent of the Company’s independent registered public accounting firm in Exhibit 15.1. No other changes have been made to the Original Filing. For the convenience of the reader, this Amendment No. 1 restates in its entirety the Original Filing. This Amendment No. 1 speaks as of the filing date of the Original Filing, does not reflect events that may have occurred subsequent to the filing date of the Original Filing, and does not modify or update in any way disclosures made in the Original Filing, except as noted above.
i
INTRODUCTION
Can-Fite is a clinical-stage biopharmaceutical company focused on developing orally bioavailable small molecule therapeutic products for the treatment of cancer, inflammatory disease and sexual dysfunction. Our platform technology utilizes the Gi protein associated A3 adenosine receptor, or A3AR, as a therapeutic target. A3AR is highly expressed in inflammatory and cancer cells, and not significantly expressed in normal cells, suggesting that the receptor could be a unique target for pharmacological intervention. Our pipeline of drug candidates are synthetic, highly specific agonists and allosteric modulators, or ligands or molecules that initiate molecular events when binding with target proteins, targeting the A3AR.
Our ordinary shares have been trading on the Tel Aviv Stock Exchange, or TASE, under the symbol “CFBI” since October 2005. On October 2, 2012, our ADSs began trading over the counter, or OTC, in the United States under the symbol “CANFY” and on November 19, 2013, our ADSs began trading on the NYSE American under the symbol “CANF.”
Unless otherwise indicated, all references to the “Company,” “we,” “our” and “Can-Fite” refer to Can-Fite BioPharma Ltd. and its consolidated subsidiaries. References to “ordinary shares”, “ADSs”, “warrants” and “share capital” refer to the ordinary shares, ADSs, warrants and share capital, respectively, of Can-Fite.
References to “U.S. dollars”, “USD”, and “$” are to currency of the United States of America, and references to “NIS” are to New Israeli Shekels. References to “ordinary shares” are to our ordinary shares, par value of NIS 0.25 per share. We report financial information under International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or the IASB, and none of the financial statements were prepared in accordance with generally accepted accounting principles in the United States.
Unless otherwise indicated, U.S. dollar translations of NIS amounts presented in this Annual Report on Form 20-F for the year ended on December 31, 2018 are translated using the rate of NIS 3.748 to $1.00, the exchange rate reported by the Bank of Israel on December 31, 2018, U.S. dollar translations of NIS amounts presented in this Annual Report on Form 20-F for the year ended on December 31, 2017 are translated using the rate of NIS 3.467 to $1.00, the exchange rate reported by the Bank of Israel on December 31, 2017, and U.S. dollar translations of NIS amounts presented in this Annual Report on Form 20-F for the year ended on December 31, 2016 are translated using the rate of NIS 3.845 to $1.00, the exchange rate reported by the Bank of Israel on December 31, 2016.
ii
FORWARD LOOKING STATEMENTS
This Annual Report on Form 20-F contains forward-looking statements, about our expectations, beliefs or intentions regarding, among other things, our product development efforts, business, financial condition, results of operations, strategies or prospects. In addition, from time to time, we or our representatives have made or may make forward-looking statements, orally or in writing. Forward-looking statements can be identified by the use of forward-looking words such as “believe,” “expect,” “intend,” “plan,” “may,” “should” or “anticipate” or their negatives or other variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical or current matters. These forward-looking statements may be included in, but are not limited to, various filings made by us with the U.S. Securities and Exchange Commission, or the SEC, press releases or oral statements made by or with the approval of one of our authorized executive officers. Forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause our actual results to differ materially from any future results expressed or implied by the forward-looking statements. Many factors could cause our actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including, but not limited to, the factors summarized below.
This Annual Report on Form 20-F identifies important factors which could cause our actual results to differ materially from those indicated by the forward-looking statements, particularly those set forth under the heading “Risk Factors.” The risk factors included in this Annual Report on Form 20-F are not necessarily all of the important factors that could cause actual results to differ materially from those expressed in any of our forward-looking statements. Given these uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements. Factors that could cause our actual results to differ materially from those expressed or implied in such forward-looking statements include, but are not limited to:
| ● | our history of losses and needs for additional capital to fund our operations and our inability to obtain additional capital on acceptable terms, or at all; | |
| ● | uncertainties of cash flows and inability to meet working capital needs; | |
| ● | the initiation, timing, progress and results of our preclinical studies, clinical trials and other product candidate development efforts; |
| ● | our ability to advance our product candidates into clinical trials or to successfully complete our preclinical studies or clinical trials; |
| ● | our receipt of regulatory approvals for our product candidates, and the timing of other regulatory filings and approvals; |
| ● | the clinical development, commercialization and market acceptance of our product candidates; |
| ● | our ability to establish and maintain strategic partnerships and other corporate collaborations; |
| ● | the implementation of our business model and strategic plans for our business and product candidates; |
| ● | the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and our ability to operate our business without infringing the intellectual property rights of others; |
| ● | competitive companies, technologies and our industry; and |
| ● | statements as to the impact of the political and security situation in Israel on our business. |
All forward-looking statements attributable to us or persons acting on our behalf speak only as of the date of this Annual Report on Form 20-F and are expressly qualified in their entirety by the cautionary statements included in this Annual Report on Form 20-F. We undertake no obligations to update or revise forward-looking statements to reflect events or circumstances that arise after the date made or to reflect the occurrence of unanticipated events. In evaluating forward-looking statements, you should consider these risks and uncertainties.
EXPLANATORY NOTE
Market data and certain industry data and forecasts used throughout this Annual Report on Form 20-F were obtained from internal company surveys, market research, consultant surveys, publicly available information, reports of governmental agencies and industry publications and surveys. Industry surveys, publications, consultant surveys and forecasts generally state that the information contained therein has been obtained from sources believed to be reliable, but that the accuracy and completeness of such information is not guaranteed. We have not independently verified any of the data from third-party sources, nor have we ascertained the underlying economic assumptions relied upon therein. Similarly, internal surveys, industry forecasts and market research, which we believe to be reliable based upon our management’s knowledge of the industry, have not been independently verified. Forecasts are particularly likely to be inaccurate, especially over long periods of time. In addition, we do not necessarily know what assumptions regarding general economic growth were used in preparing the forecasts we cite. Statements as to our market position are based on the most currently available data. While we are not aware of any misstatements regarding the industry data presented in this Annual Report on Form 20-F, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” in this Annual Report on Form 20-F.
iii
ITEM 1. Identity of Directors, Senior Management and Advisers.
Not applicable.
ITEM 2. Offer Statistics and Expected Timetable.
Not applicable.
A. Selected Financial Data.
The following table sets forth our selected consolidated financial data for the periods ended and as of the dates indicated. The following selected consolidated financial data for our company should be read in conjunction with the financial information, “Item 5. Operating and Financial Review and Prospects” and other information provided elsewhere in this Annual Report on Form 20-F and our consolidated financial statements and related notes. The selected consolidated financial data in this section is not intended to replace the consolidated financial statements and is qualified in its entirety thereby.
The selected consolidated statements of operations data for the years ended December 31, 2018, 2017 and 2016, and the selected consolidated balance sheet data as of December 31, 2018 and 2017, have been derived from our audited consolidated financial statements set forth elsewhere in this Annual Report on Form 20-F. The selected consolidated statements of operations data for the years ended December 31, 2015 and 2014, and the selected consolidated balance sheet data as of December 31, 2016, 2015 and 2014, have been derived from our audited consolidated financial statements not included in this Form 20-F.
Our consolidated financial statements included in this Annual Report on Form 20-F were prepared in accordance with IFRS as issued by the IASB.
From our inception through January 1, 2018, our functional and presentation currency was the New Israeli Shekel, or NIS. Effective January 1, 2018, our functional and reporting currency is the U.S. dollar which is the primary currency of the economic environment in which we operate. Due to the change in our functional and reporting currency from the NIS to the U.S. dollar, effective January 1, 2018, the amounts for 2015 have been restated in U.S. dollars using the methodology set forth in Note 2d to our consolidated financial statements for the year ended December 31, 2018.
| Year Ended December 31, | ||||||||||||||||||||
| Consolidated Statements Of Operations Data: | 2014 | 2015 | 2016 | 2017 | 2018 | |||||||||||||||
| (USD in thousands, except share and per share data) | ||||||||||||||||||||
| Revenues | - | 162 | 165 | 789 | 3,820 | |||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Research and development expenses, net | 4,536 | 3,904 | 6,115 | 5,106 | 6,075 | |||||||||||||||
| General and administrative expenses | 3,099 | 2,735 | 2,733 | 2,868 | 3,159 | |||||||||||||||
| Operating loss | 7,635 | 6,477 | 8,683 | 7,185 | 5,414 | |||||||||||||||
| Other income | - | - | - | (769 | ) | - | ||||||||||||||
| Financial expenses | 17 | 133 | 55 | 621 | 1,204 | |||||||||||||||
| Financial income | (618 | ) | (106 | ) | (374 | ) | (633 | ) | (51 | ) | ||||||||||
| Taxes on income | 6 | 5 | 29 | 29 | 4 | |||||||||||||||
| Net loss | 7,040 | 6,509 | 8,393 | 6,433 | 6,571 | |||||||||||||||
| Adjustments arising from translating financial statements from functional currency to presentation currency | 135 | 58 | (119 | ) | (636 | ) | - | |||||||||||||
| Remeasurements loss (gain) from defined benefit plan | 27 | 99 | - | - | - | |||||||||||||||
| Comprehensive loss | 7,202 | 6,666 | 8,274 | 5,797 | 6,571 | |||||||||||||||
| Net loss per ordinary share | 0.39 | 0.27 | 0.30 | 0.19 | 0.17 | |||||||||||||||
| Number of ordinary shares used in computing loss per ordinary share | 17,545,663 | 22,953,077 | 27,692,668 | 32,525,138 | 38,902,214 | |||||||||||||||
1
| As of December 31, | ||||||||||||||||||||
| Consolidated Balance Sheet Data: | 2014 | 2015 | 2016 | 2017 | 2018 | |||||||||||||||
| USD in thousands | ||||||||||||||||||||
| Cash and cash equivalents | 9,280 | 16,921 | 8,115 | 3,505 | 3,615 | |||||||||||||||
| Other receivables and lease deposit | 946 | 657 | 2,017 | 3,164 | 4,017 | |||||||||||||||
| Short-term investment | - | - | - | - | 273 | |||||||||||||||
| long-term investments | - | - | - | 917 | - | |||||||||||||||
| Fixed assets | 22 | 48 | 40 | 28 | 47 | |||||||||||||||
| Total assets | 10,248 | 17,626 | 10,172 | 7,614 | 7,952 | |||||||||||||||
| Total liabilities | 2,276 | 3,698 | 4,211 | 2,600 | 4,937 | |||||||||||||||
| Total shareholders’ equity | 7,972 | 13,928 | 5,961 | 5,014 | 3,015 | |||||||||||||||
B. Capitalization and Indebtedness.
Not applicable.
C. Reasons for the Offer and Use of Proceeds.
Not applicable.
D. Risk Factors
You should carefully consider the risks we describe below, in addition to the other information set forth elsewhere in this Annual Report on Form 20-F, including our consolidated financial statements and the related notes beginning on page F-1, before deciding to invest in our ordinary shares and American Depositary Shares, or ADSs. These material risks could adversely impact our results of operations, possibly causing the trading price of our ordinary shares and ADSs to decline, and you could lose all or part of your investment.
Risks Related to Our Financial Position and Capital Requirements
We have incurred operating losses since our inception and anticipate that we will continue to incur substantial operating losses for the foreseeable future.
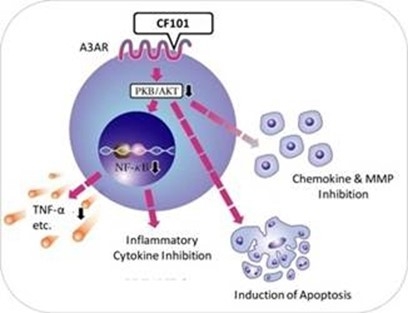
We are a clinical stage biopharmaceutical company that develops orally bioavailable small molecule therapeutic products for the treatment of cancer, liver and inflammatory diseases and sexual dysfunction. Since our incorporation in 1994, we have been focused on research and development activities with a view to developing our product candidates, CF101, also known as Piclidenoson, CF102, also known as Namodenoson, and CF602. We have financed our operations primarily through the sale of equity securities (both in private placements and in public offerings on the TASE and NYSE American) and payments received under out-licensing agreements and have incurred losses in each year since our inception in 1994. We have historically incurred substantial net losses, including net losses of approximately $6.5 million in 2018, $6.4 million in 2017, and $8.4 million in 2016. As of December 31, 2018, we had an accumulated deficit of approximately $100.6 million. We do not know whether or when we will become profitable. To date, we have not commercialized any products or generated any revenues from product sales and accordingly we do not have a revenue stream to support our cost structure. Our losses have resulted principally from costs incurred in development and discovery activities. We expect to continue to incur losses for the foreseeable future, and these losses will likely increase as we:
| ● | initiate and manage pre-clinical development and clinical trials for our current and new product candidates; |
| ● | seek regulatory approvals for our product candidates; |
2
| ● | implement internal systems and infrastructures; |
| ● | seek to license additional technologies to develop; |
| ● | hire management and other personnel; and |
| ● | move towards commercialization. |
If our product candidates fail in clinical trials or do not gain regulatory clearance or approval, or if our product candidates do not achieve market acceptance, we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our inability to achieve and then maintain profitability would negatively affect our business, financial condition, results of operations and cash flows. Moreover, our prospects must be considered in light of the risks and uncertainties encountered by an early-stage company and in highly regulated and competitive markets, such as the biopharmaceutical market, where regulatory approval and market acceptance of our products are uncertain. There can be no assurance that our efforts will ultimately be successful or result in revenues or profits.
We will need to raise additional capital to meet our business requirements in the future, and such capital raising may be costly or difficult to obtain and will dilute current shareholders’ ownership interests.
As of December 31, 2018 we had cash and cash equivalents of $3.6 million. In January 2019 we raised approximately $2.35 million in a registered direct offering and concurrent private placement. We believe that our existing financial resources will be sufficient to meet our requirements for the next twelve months from the date of issuance of this Annual Report on Form 20-F. We have expended and believe that we will continue to expend substantial resources for the foreseeable future developing our product candidates. These expenditures will include costs associated with research and development, manufacturing, conducting preclinical experiments and clinical trials and obtaining regulatory approvals, as well as commercializing any products approved for sale. Because the outcome of our planned and anticipated clinical trials is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of our product candidates. In addition, other unanticipated costs may arise. As a result of these and other factors currently unknown to us, we will require additional funds, through public or private equity or debt financings or other sources, such as strategic partnerships and alliances and licensing arrangements. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Our future capital requirements will depend on many factors, including the progress and results of our clinical trials, the duration and cost of discovery and preclinical development, and laboratory testing and clinical trials for our product candidates, the timing and outcome of regulatory review of our product candidates, the number and development requirements of other product candidates that we pursue, and the costs of activities, such as product marketing, sales, and distribution. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our anticipated clinical trials.
3
Our future capital requirements depend on many factors, including:
| ● | the level of research and development investment required to develop our product candidates; | |
| ● | the failure to obtain regulatory approval or achieve commercial success of our product candidates, including Piclidenoson, Namodenoson and CF602; |
| ● | the results of our preclinical studies and clinical trials for our earlier stage product candidates, and any decisions to initiate clinical trials if supported by the preclinical results; |
| ● | the costs, timing and outcome of regulatory review of our product candidates that progress to clinical trials; |
| ● | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our issued patents and defending intellectual property-related claims; |
| ● | the cost of commercialization activities if any of our product candidates are approved for sale, including marketing, sales and distribution costs; |
| ● | the cost of manufacturing our product candidates and any products we successfully commercialize; |
| ● | the timing, receipt and amount of sales of, or royalties on, our future products, if any; |
| ● | the expenses needed to attract and retain skilled personnel; |
| ● | any product liability or other lawsuits related to our products; |
| ● | the extent to which we acquire or invest in businesses, products or technologies and other strategic relationships; |
| ● | the costs of financing unanticipated working capital requirements and responding to competitive pressures; and | |
| ● | maintaining minimum shareholders’ equity requirements and complying with other continue listing standards under the NYSE American Company Guide. |
Additional funds may not be available when we need them, on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate preclinical studies, clinical trials or other research and development activities for one or more of our product candidates or delay, limit, reduce or terminate our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize our product candidates.
We may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we issue, such as convertible notes and warrants, which may adversely impact our financial condition.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
We may seek additional capital through a combination of private and public equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of existing shareholders will be diluted, and the terms may include liquidation or other preferences that adversely affect shareholder rights. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take certain actions, such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us. If we are unable to raise additional funds through equity or debt financing when needed, we may be required to delay, limit, reduce or terminate our product development or commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
4
Risks Related to Our Business and Regulatory Matters
We have not yet commercialized any products or technologies, and we may never become profitable.
We have not yet commercialized any products or technologies, and we may never be able to do so. We do not know when or if we will complete any of our product development efforts, obtain regulatory approval for any product candidates incorporating our technologies or successfully commercialize any approved products. Even if we are successful in developing products that are approved for marketing, we will not be successful unless these products gain market acceptance for appropriate indications at favorable reimbursement rates. The degree of market acceptance of these products will depend on a number of factors, including:
| ● | the timing of regulatory approvals in the countries, and for the uses, we seek; |
| ● | the competitive environment; |
| ● | the establishment and demonstration in the medical community of the safety and clinical efficacy of our products and their potential advantages over existing therapeutic products; |
| ● | our ability to enter into distribution and other strategic agreements with pharmaceutical and biotechnology companies with strong marketing and sales capabilities; |
| ● | the adequacy and success of distribution, sales and marketing efforts; and |
| ● | the pricing and reimbursement policies of government and third-party payors, such as insurance companies, health maintenance organizations and other plan administrators. |
Physicians, patients, thirty-party payors or the medical community in general may be unwilling to accept, utilize or recommend, and in the case of third-party payors, cover any of our products or products incorporating our technologies. As a result, we are unable to predict the extent of future losses or the time required to achieve profitability, if at all. Even if we successfully develop one or more products that incorporate our technologies, we may not become profitable.
Our product candidates are at various stages of clinical and preclinical development and may never be commercialized.
Our product candidates are at various stages of clinical development and may never be commercialized. The progress and results of any future pre-clinical testing or future clinical trials are uncertain, and the failure of our product candidates to receive regulatory approvals will have a material adverse effect on our business, operating results and financial condition to the extent we are unable to commercialize any products. None of our product candidates has received regulatory approval for commercial sale. In addition, we face the risks of failure inherent in developing therapeutic products. Our product candidates are not expected to be commercially available for several years, if at all.
In addition, our product candidates must satisfy rigorous standards of safety and efficacy before they can be approved by the U.S. Food and Drug Administration, or the FDA, the European Medicines Agency, or the EMA, and foreign regulatory authorities for commercial use. The FDA, the EMA and foreign regulatory authorities have full discretion over this approval process. We will need to conduct significant additional research, involving testing in animals and in humans, before we can file applications for product approval. Typically, in the pharmaceutical industry, there is a high rate of attrition for product candidates in pre-clinical testing and clinical trials. Also, satisfying regulatory requirements typically takes many years, is dependent upon the type, complexity and novelty of the product and requires the expenditure of substantial resources. In addition, delays or rejections may be encountered based upon additional government regulation, including any changes in FDA policy, during the process of product development, clinical trials and regulatory reviews.
5
In order to receive FDA approval or approval from foreign regulatory authorities to market a product candidate or to distribute our products, we must demonstrate thorough pre-clinical testing and thorough human clinical trials that the product candidate is safe and effective for its intended uses (e.g., treatment of a specific condition in a specific way subject to contradictions and other limitations). Even if we comply with all FDA requests, the FDA may ultimately reject one or more of our New Drug Applications, or NDA, or grant approval for a narrowly intended use that is not commercially feasible. We might not obtain regulatory approval for our drug candidates in a timely manner, if at all. Failure to obtain FDA approval of any of our drug candidates in a timely manner or at all will severely undermine our business by reducing the number of salable products and, therefore, corresponding product revenues.
Results of earlier clinical trials may not be predictive of the results of later-stage clinical trials.
The results of preclinical studies and early clinical trials of product candidates may not be predictive of the results of later-stage clinical trials. Also, interim results, if at all, during a clinical trial do not necessarily predict final results. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy results despite having progressed through preclinical studies and initial clinical trials. For example, in December 2013, our former subsidiary OphthaliX Inc. (since renamed Wize Pharma, Inc.), or OphthaliX, announced top-line results of a Phase III study with Piclidenoson for dry-eye syndrome in which Piclidenoson did not meet the primary efficacy endpoint of complete clearing of corneal staining, nor the secondary efficacy endpoints and in July 2016, OphthaliX released top-line results from its Phase II clinical trial of Piclidenoson for the treatment of glaucoma in which no statistically significant differences were found between the Piclidenoson treated group and the placebo group in the primary endpoint of lowering intraocular pressure, or IOP. In addition, two Phase IIb studies in rheumatoid arthritis, or rheumatoid arthritis, utilizing Piclidenoson in combination with methotrexate, a generic drug commonly used for treating rheumatoid arthritis patients, or MTX, failed to reach their primary endpoints. A Phase II/III study of Piclidenoson for psoriasis did not meet its primary endpoint although positive data from further analysis of the Phase II/III study suggests Piclidenoson as a potential systemic therapy for patients with moderate-severe psoriasis. Furthermore, a Phase II study for advanced HCC in subjects with Child-Pugh B who failed Nexavar as a first line treatment did not meet its primary endpoint although it showed superiority in overall survival in the largest study subpopulation.
Many companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials due to adverse safety profiles or lack of efficacy, notwithstanding promising results in earlier studies. Any delay in, or termination or suspension of, our clinical trials will delay the requisite filings with the FDA, the EMA or other foreign regulatory authorities and, ultimately, our ability to commercialize our product candidates and generate product revenues. If the clinical trials do not support our product claims, the completion of development of such product candidates may be significantly delayed or abandoned, which will significantly impair our ability to generate product revenues and will materially adversely affect our results of operations.
This drug candidate development risk is heightened by any changes in the planned clinical trials compared to the completed clinical trials. As product candidates are developed from preclinical through early to late stage clinical trials towards approval and commercialization, it is customary that various aspects of the development program, such as manufacturing and methods of administration, are altered along the way in an effort to optimize processes and results. While these types of changes are common and are intended to optimize the product candidates for late stage clinical trials, approval and commercialization, such changes do carry the risk that they will not achieve these intended objectives.
Changes in our planned clinical trials or future clinical trials could cause our product candidates to perform differently, including causing toxicities, which could delay completion of our clinical trials, delay approval of our product candidates, and/or jeopardize our ability to commence product sales and generate revenues.
6
We might be unable to develop product candidates that will achieve commercial success in a timely and cost-effective manner, or ever.
Even if regulatory authorities approve our product candidates, they may not be commercially successful. Our product candidates may not be commercially successful because government agencies and other third-party payors may not cover the product or the coverage may be too limited to be commercially successful; physicians and others may not use or recommend our products, even following regulatory approval. A product approval, assuming one issues, may limit the uses for which the product may be distributed thereby adversely affecting the commercial viability of the product. Third parties may develop superior products or have proprietary rights that preclude us from marketing our products. We also expect that at least some of our product candidates will be expensive, if approved. Patient acceptance of and demand for any product candidates for which we obtain regulatory approval or license will depend largely on many factors, including but not limited to the extent, if any, of reimbursement of costs by government agencies and other third-party payors, pricing, the effectiveness of our marketing and distribution efforts, the safety and effectiveness of alternative products, and the prevalence and severity of side effects associated with our products. If physicians, government agencies and other third-party payors do not accept our products, we will not be able to generate significant revenue.
Our current pipeline is based on our platform technology utilizing the Gi protein associated A3AR, as a potent therapeutic target and currently includes three molecules, Piclidenoson, Namodenoson and CF602 product candidates, of which Piclidenoson is the most advanced. Failure to develop these molecules will have a material adverse effect on us.
Our current pipeline is based on a platform technology where we target the A3AR with highly selective ligands, or small signal triggering molecules that bind to specific cell surface receptors, such as the A3AR, including Piclidenoson, Namodenoson and CF602. A3ARs are structures found in cell surfaces that record and transfer messages from small molecules or ligands, such as Piclidenoson, Namodenoson and CF602 to the rest of the cell. Piclidenoson is the most advanced of our drug candidates. As such, we are currently dependent on only three molecules for our potential commercial success, and any safety or efficacy concerns related to such molecules would have a significant impact on our business. Failure to develop our drug candidates, in whole or in part, will have a material adverse effect on us.
Clinical trials are very expensive, time-consuming and difficult to design and implement, and, as a result, we may suffer delays or suspensions in future trials which would have a material adverse effect on our ability to generate revenues.
Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. Regulatory authorities, such as the FDA, may preclude clinical trials from proceeding. Additionally, the clinical trial process is time-consuming, failure can occur at any stage of the trials, and we may encounter problems that cause us to abandon or repeat clinical trials. The commencement and completion of clinical trials may be delayed by several factors, including:
| ● | unforeseen safety issues; |
| ● | determination of dosing issues; |
| ● | lack of effectiveness or efficacy during clinical trials; |
| ● | failure of third-party suppliers to perform final manufacturing steps for the drug substance; |
| ● | slower than expected rates of patient recruitment and enrollment; |
| ● | lack of healthy volunteers and patients to conduct trials; |
| ● | inability to monitor patients adequately during or after treatment; |
| ● | failure of third-party contract research organizations to properly implement or monitor the clinical trial protocols; |
7
| ● | failure of institutional review boards to approve our clinical trial protocols; |
| ● | inability or unwillingness of medical investigators and institutional review boards to follow our clinical trial protocols; and |
| ● | lack of sufficient funding to finance the clinical trials. |
We have experienced the risks involved with conducting clinical trials, including but not limited to, increased expense and delay and failure to meet end points of the trial. For example, in December 2013, OphthaliX, announced top-line results of a Phase III study with CF101 for dry-eye syndrome in which Piclidenoson did not meet the primary efficacy endpoint of complete clearing of corneal staining, nor the secondary efficacy endpoints and in July 2016, OphthaliX released top-line results from its Phase II clinical trial of Piclidenoson for the treatment of glaucoma in which no statistically significant differences were found between the Piclidenoson treated group and the placebo group in the primary endpoint of lowering IOP. In addition, two Phase IIb studies in rheumatoid arthritis, utilizing Piclidenoson in combination with MTX failed to reach their primary end points. A Phase II/III study of Piclidenoson for psoriasis did not meet its primary endpoint although positive data from further analysis of the Phase II/III study suggests Piclidenoson as a potential systemic therapy for patients with moderate-severe psoriasis. Furthermore, a Phase II study for advanced HCC in subjects with Child-Pugh B who failed Nexavar as a first line treatment did not meet its primary endpoint although it showed superiority in overall survival in the largest study subpopulation.
In addition, we or regulatory authorities may suspend our clinical trials at any time if it appears that we are exposing participants to unacceptable health risks or if the regulatory authorities find deficiencies in our regulatory submissions or the conduct of these trials. Any suspension of clinical trials will delay possible regulatory approval, if any, and adversely impact our ability to develop products and generate revenue.
If we acquire or license additional technology or product candidates, we may incur a number of costs, may have integration difficulties and may experience other risks that could harm our business and results of operations.
We may acquire and license additional product candidates and technologies. Any product candidate or technology we license from others or acquire will likely require additional development efforts prior to commercial sale, including extensive pre-clinical or clinical testing, or both, and approval by the FDA and applicable foreign regulatory authorities, if any. All product candidates are prone to risks of failure inherent in pharmaceutical product development, including the possibility that the product candidate or product developed based on licensed technology will not be shown to be sufficiently safe and effective for approval by regulatory authorities. In addition, we cannot assure you that any product candidate that we develop based on acquired or licensed technology that is granted regulatory approval will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace. Moreover, integrating any newly acquired product candidates could be expensive and time-consuming. If we cannot effectively manage these aspects of our business strategy, our business may not succeed.
The manufacture of our product candidates is a chemical synthesis process and if one of our materials suppliers encounters problems manufacturing our products, our business could suffer.
The FDA and foreign regulators require manufacturers to register manufacturing facilities. The FDA and foreign regulators also inspect these facilities to confirm compliance with requirements that the FDA or foreign regulators establish. We do not intend to engage in the manufacture of our products other than for pre-clinical and clinical studies, but we or our materials suppliers may face manufacturing or quality control problems causing product production and shipment delays or a situation where we or the supplier may not be able to maintain compliance with the FDA’s or foreign regulators’ requirements necessary to continue manufacturing our drug substance. Drug manufacturers are subject to ongoing periodic unannounced inspections by the FDA, the U.S. Drug Enforcement Agency, or DEA, and corresponding foreign regulators to ensure strict compliance with requirements and other governmental regulations and corresponding foreign standards. Any failure to comply with DEA requirements or FDA or foreign regulatory requirements could adversely affect our clinical research activities and our ability to market and develop our product candidates.
8
We do not currently have sales, marketing or distribution capabilities or experience, and we are unable to effectively sell, market or distribute our product candidates now and we do not expect to be able to do so in the future. The failure to enter into agreements with third parties that are capable of performing these functions would have a material adverse effect on our business and results of operations.
We do not currently have and we do not expect to develop sales, marketing and distribution capabilities. If we are unable to enter into agreements with third parties to perform these functions, we will not be able to successfully market any of our platforms or product candidates. In order to successfully market any of our platform or product candidates, we must make arrangements with third parties to perform these services.
As we do not intend to develop a marketing and sales force with technical expertise and supporting distribution capabilities, we will be unable to market any of our product candidates directly. To promote any of our potential products through third parties, we will have to locate acceptable third parties for these functions and enter into agreements with them on acceptable terms, and we may not be able to do so. Any third-party arrangements we are able to enter into may result in lower revenues than we could achieve by directly marketing and selling our potential products. In addition, to the extent that we depend on third parties for marketing and distribution, any revenues we receive will depend upon the efforts of such third parties, as well as the terms of our agreements with such third parties, which cannot be predicted in most cases at this time. As a result, we might not be able to market and sell our products in the United States or overseas, which would have a material adverse effect on us.
We will to some extent rely on third parties to implement our manufacturing and supply strategies. Failure of these third parties in any respect could have a material adverse effect on our business, results of operations and financial condition.
If our current and future manufacturing and supply strategies are unsuccessful, then we may be unable to conduct and complete any future pre-clinical or clinical trials or commercialize our product candidates in a timely manner, if at all. Completion of any potential future pre-clinical or clinical trials and commercialization of our product candidates will require access to, or development of, facilities to manufacture a sufficient supply of our product candidates. We do not have the resources, facilities or experience to manufacture our product candidates for commercial purposes on our own and do not intend to develop or acquire facilities for the manufacture of product candidates for commercial purposes in the foreseeable future. We may rely on contract manufacturers to produce sufficient quantities of our product candidates necessary for any pre-clinical or clinical testing we undertake in the future. Such contract manufacturers may be the sole source of production and they may have limited experience at manufacturing, formulating, analyzing, filling and finishing our types of product candidates.
We also intend to rely on third parties to supply the requisite materials needed for the manufacturing of our active pharmaceutical ingredients, or API. There may be a limited supply of these requisite materials. We might not be able to enter into agreements that provide us assurance of availability of such components in the future from any supplier. Our potential suppliers may not be able to adequately supply us with the components necessary to successfully conduct our pre-clinical and clinical trials or to commercialize our product candidates. If we cannot acquire an acceptable supply of the requisite materials to produce our product candidates, we will not be able to complete pre-clinical and clinical trials and will not be able to market or commercialize our product candidates.
We depend on key members of our management and key consultants and will need to add and retain additional leading experts. Failure to retain our management and consulting team and add additional leading experts could have a material adverse effect on our business, results of operations or financial condition.
We are highly dependent on our executive officers and other key management and technical personnel. Our failure to retain our Chief Executive Officer, Pnina Fishman, Ph.D., who has developed much of the technology we utilize today, or any other key management and technical personnel, could have a material adverse effect on our future operations. Our success is also dependent on our ability to attract, retain and motivate highly trained technical, and management personnel, among others, to continue the development and commercialization of our current and future products. We presently maintain a life insurance policy on our Chief Executive Officer, Pnina Fishman, Ph.D.
9
Our success also depends on our ability to attract, retain and motivate personnel required for the development, maintenance and expansion of our activities. There can be no assurance that we will be able to retain our existing personnel or attract additional qualified employees or consultants. The loss of key personnel or the inability to hire and retain additional qualified personnel in the future could have a material adverse effect on our business, financial condition and results of operation.
We face significant competition and continuous technological change, and developments by competitors may render our products or technologies obsolete or non-competitive. If we cannot successfully compete with new or existing products, our marketing and sales will suffer and we may not ever be profitable.
We will compete against fully integrated pharmaceutical and biotechnology companies and smaller companies that are collaborating with larger pharmaceutical companies, academic institutions, government agencies and other public and private research organizations. In addition, many of these competitors, either alone or together with their collaborative partners, operate larger research and development programs than we do, and have substantially greater financial resources than we do, as well as significantly greater experience in:
| ● | developing drugs; |
| ● | undertaking pre-clinical testing and human clinical trials; |
| ● | obtaining FDA approval, addressing various regulatory matters and other regulatory approvals of drugs; |
| ● | formulating and manufacturing drugs; and |
| ● | launching, marketing and selling drugs. |
If our competitors develop and commercialize products faster than we do, or develop and commercialize products that are superior to our product candidates, our commercial opportunities will be reduced or eliminated. The extent to which any of our product candidates achieve market acceptance will depend on competitive factors, many of which are beyond our control. Competition in the biotechnology and biopharmaceutical industry is intense and has been accentuated by the rapid pace of technology development. Our competitors include large integrated pharmaceutical companies, biotechnology companies that currently have drug and target discovery efforts, universities, and public and private research institutions. Almost all of these entities have substantially greater research and development capabilities and financial, scientific, manufacturing, marketing and sales resources than we do. These organizations also compete with us to:
| ● | attract parties for acquisitions, joint ventures or other collaborations; |
| ● | license proprietary technology that is competitive with the technology we are developing; |
| ● | attract funding; and |
| ● | attract and hire scientific talent and other qualified personnel. |
Our competitors may succeed in developing and commercializing products earlier and obtaining regulatory approvals from the FDA or foreign regulators more rapidly than we do. Our competitors may also develop products or technologies that are superior to those we are developing, and render our product candidates or technologies obsolete or non-competitive. If we cannot successfully compete with new or existing products, our marketing and sales will suffer and we may not ever be profitable.
Our competitors currently include companies with marketed products and/or an advanced research and development pipeline. The major competitors in the rheumatoid arthritis and psoriasis therapeutic field include Amgen, J&J, Pfizer, Novartis, Abbvie, Celgene, Eli Lilly, Bristol-Myers, and more. Competitors in the HCC field include companies such as Bayer, Exelixis, Merck, and Bristol-Myers. Competitors in the NASH field include companies such as Gilead, Genfit, Galmed, Allergan, Intercept, and Madrigal. Competitors in the erectile dysfunction field include Pfizer, Eli Lilly and Bayer. See “Item 4. Information on the Company—B. Business Overview—Competition.”
10
Moreover, several companies have reported the commencement of research projects related to the A3AR. Such companies include CV Therapeutics Inc. (which was acquired by Gilead), King Pharmaceuticals R&D Inv. (which was acquired by Pfizer), Hoechst Marion Roussel Inc. (which was acquired by Aventis), Novo Nordisk A/S and Inotek Pharmaceuticals. However, to the best of our knowledge, there is no approved drug currently on the market, which is similar to our A3AR agonists, nor are we aware of any allosteric modulator in the A3AR product pipeline similar to our allosteric modulator with respect to chemical profile and mechanism of action.
We may suffer losses from product liability claims if our product candidates cause harm to patients.
Any of our product candidates could cause adverse events. Although data from a pooled analysis of approximately 1,200 patients with inflammatory disease treated with Piclidenoson, indicates that Piclidenoson has a good safety profile and is well tolerated at doses up to 4.0 mg administered twice daily for up to 12-32 weeks, there were incidences (albeit less than or equal to 5%) of adverse events in eight completed and fully analyzed trials in inflammatory disease. Such adverse events included nausea, diarrhea, abdominal pain, vomiting, constipation, common bacterial and viral syndromes (such as tonsillitis, otitis and respiratory and urinary tract infections), abdominal pain, vomiting, myalgia, arthralgia, dizziness, headache and pruritus. We observed an even lower incidence (less than or equal to 2%) of serious adverse events, although only one type of event was reported in more than a single Piclidenoson-treated subject, which was exacerbation of chronic obstructive lung disease reported in two subjects. Notwithstanding the foregoing, the placebo group in such studies had a higher incidence of overall adverse events than the pooled Piclidenoson groups. In addition, in normal volunteers, Piclidenoson at doses 3-4-fold higher than those to be used in therapeutic trials, but not at therapeutic doses, was associated with prolongation of the electrocardiographic QT intervals. No new safety concerns have been identified and no novel or unexpected safety concerns have appeared over 32 weeks of treatment in more recent trials.
There is also a risk that certain adverse events may not be observed in clinical trials, but may nonetheless occur in the future. If any of these adverse events occur, they may render our product candidates ineffective or harmful in some patients, and our sales would suffer, materially adversely affecting our business, financial condition and results of operations.
In addition, potential adverse events caused by our product candidates could lead to product liability lawsuits. If product liability lawsuits are successfully brought against us, we may incur substantial liabilities and may be required to limit the marketing and commercialization of our product candidates. Our business exposes us to potential product liability risks, which are inherent in the testing, manufacturing, marketing and sale of pharmaceutical products. We may not be able to avoid product liability claims. Product liability insurance for the pharmaceutical and biotechnology industries is generally expensive, if available at all. If, at any time, we are unable to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims, we may be unable to clinically test, market or commercialize our product candidates. A successful product liability claim brought against us in excess of our insurance coverage, if any, may cause us to incur substantial liabilities, and, as a result, our business, liquidity and results of operations would be materially adversely affected.
Our product candidates will remain subject to ongoing regulatory requirements even if they receive marketing approval, and if we fail to comply with these requirements, we could lose these approvals, and the sales of any approved commercial products could be suspended.
Even if we receive regulatory approval to market a particular product candidate, the product will remain subject to extensive regulatory requirements, including requirements relating to manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion, distribution and recordkeeping. Even if regulatory approval of a product is granted, the approval may be subject to limitations on the uses for which the product may be marketed or the conditions of approval, or may contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product, which could negatively impact us or our collaboration partners by reducing revenues or increasing expenses, and cause the approved product candidate not to be commercially viable. In addition, as clinical experience with a drug expands after approval, typically because it is used by a greater number and more diverse group of patients after approval than during clinical trials, side effects and other problems may be observed after approval that were not seen or anticipated during pre-approval clinical trials or other studies. Any adverse effects observed after the approval and marketing of a product candidate could result in limitations on the use of or withdrawal of any approved products from the marketplace. Absence of long-term safety data may also limit the approved uses of our products, if any. If we fail to comply with the regulatory requirements of the FDA and other applicable U.S. and foreign regulatory authorities, or previously unknown problems with any approved commercial products, manufacturers or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions or other setbacks, including the following:
| ● | Restrictions on the products, manufacturers or manufacturing process; |
| ● | Warning letters; |
11
| ● | Civil or criminal penalties, fines and injunctions; |
| ● | Product seizures or detentions; |
| ● | Import or export bans or restrictions; |
| ● | Voluntary or mandatory product recalls and related publicity requirements; |
| ● | Suspension or withdrawal of regulatory approvals; |
| ● | Total or partial suspension of production; and |
| ● | Refusal to approve pending applications for marketing approval of new products or supplements to approved applications. |
If we or our collaborators are slow or unable to adapt to changes in existing regulatory requirements or adoption of new regulatory requirements or policies, marketing approval for our product candidates may be lost or cease to be achievable, resulting in decreased revenue from milestones, product sales or royalties, which would have a material adverse effect on our results of operations.
We deal with hazardous materials and must comply with environmental, health and safety laws and regulations, which can be expensive and restrict how we do business.
Our activities and those of our third-party manufacturers on our behalf involve the controlled storage, use and disposal of hazardous materials, including corrosive, explosive and flammable chemicals and other hazardous compounds. We and our manufacturers are subject to U.S. federal, state, and local, and Israeli and other foreign laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental contamination or injury from these materials. In addition, if we develop a manufacturing capacity, we may incur substantial costs to comply with environmental regulations and would be subject to the risk of accidental contamination or injury from the use of hazardous materials in our manufacturing process.
In the event of an accident, government authorities may curtail our use of these materials and interrupt our business operations. In addition, we could be liable for any civil damages that result, which may exceed our financial resources and may seriously harm our business. Although our Israeli insurance program covers certain unforeseen sudden pollutions, we do not maintain a separate insurance policy for any of the foregoing types of risks. In addition, although the general liability section of our life sciences policy covers certain unforeseen, sudden environmental issues, pollution in the United States and Canada is excluded from the policy. In the event of environmental discharge or contamination or an accident, we may be held liable for any resulting damages, and any liability could exceed our resources. In addition, we may be subject to liability and may be required to comply with new or existing environmental laws regulating pharmaceuticals or other medical products in the environment.
12
Our business and operations may be materially adversely affected in the event of computer system failures or security breaches.
Despite the implementation of security measures, our internal computer systems, and those of our contract research organizations, or CROs, and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, cyber-attacks, natural disasters, fire, terrorism, war, and telecommunication and electrical failures. If such an event were to occur and interrupt our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, loss of trade secrets or inappropriate disclosure of confidential or proprietary information, including protected health information or personal data of employees or former employees, access to our clinical data, or disruption of the manufacturing process, we could incur liability and the further development of our drug candidates could be delayed. We may also be vulnerable to cyber-attacks by hackers or other malfeasance. This type of breach of our cybersecurity may compromise our confidential information and/or our financial information and adversely affect our business or result in legal proceedings. Further, these cybersecurity breaches may inflict reputational harm upon us that may result in decreased market value and erode public trust.
We may not be able to successfully grow and expand our business. Failure to manage our growth effectively will have a material adverse effect on our business, results of operations and financial condition.
We may not be able to successfully grow and expand. Successful implementation of our business plan will require management of growth, including potentially rapid and substantial growth, which will result in an increase in the level of responsibility for management personnel and place a strain on our human and capital resources. To manage growth effectively, we will be required to continue to implement and improve our operating and financial systems and controls to expand, train and manage our employee base. Our ability to manage our operations and growth effectively requires us to continue to expend funds to enhance our operational, financial and management controls, reporting systems and procedures and to attract and retain sufficient numbers of talented personnel. If we are unable to scale up and implement improvements to our control systems in an efficient or timely manner, or if we encounter deficiencies in existing systems and controls, then we will not be able to make available the products required to successfully commercialize our technology. Failure to attract and retain sufficient numbers of talented personnel will further strain our human resources and could impede our growth or result in ineffective growth. Moreover, the management, systems and controls currently in place or to be implemented may not be adequate for such growth, and the steps taken to hire personnel and to improve such systems and controls might not be sufficient. If we are unable to manage our growth effectively, it will have a material adverse effect on our business, results of operations and financial condition.
If we are unable to obtain adequate insurance, our financial condition could be adversely affected in the event of uninsured or inadequately insured loss or damage. Our ability to effectively recruit and retain qualified officers and directors could also be adversely affected if we experience difficulty in obtaining adequate directors’ and officers’ liability insurance.
We may not be able to obtain insurance policies on terms affordable to us that would adequately insure our business and property against damage, loss or claims by third parties. To the extent our business or property suffers any damages, losses or claims by third parties, which are not covered or adequately covered by insurance, our financial condition may be materially adversely affected.
We may be unable to maintain sufficient insurance as a public company to cover liability claims made against our officers and directors. If we are unable to adequately insure our officers and directors, we may not be able to retain or recruit qualified officers and directors to manage us.
13
Risks Related to Our Intellectual Property
The termination of the National Institute of Health, or NIH, license agreement between us and NIH due to patent expiration may diminish our proprietary position.
As a result of the termination of the NIH license agreement between us and NIH in June 2015 due to patent expiration, we no longer hold rights to a family of composition of matter patents relating to Piclidenoson that were licensed from NIH. Nevertheless, because Piclidenoson may be a new chemical entity, or NCE, following approval of an NDA, we, if we are the first applicant to obtain NDA approval, may be entitled to five years of data exclusivity in the United States with respect to such NCEs. Analogous data and market exclusivity provisions, of varying duration, may be available in Europe and other foreign jurisdictions. We also have rights under our pharmaceutical use issued patents with respect to Piclidenoson and Namodenoson, which provide patent exclusivity within our field of activity until the mid- to late-2020s. While we believe that we may be able to protect our exclusivity through such use patent portfolio and such period of exclusivity, the lack of composition of matter patent protection may diminish our ability to maintain a proprietary position for our intended uses of Piclidenoson. Moreover, we cannot be certain that we will be the first applicant to obtain an FDA approval for any indication of Piclidenoson and we cannot be certain that we will be entitled to NCE exclusivity. In addition, we have discontinued the prosecution of a family of pending patent applications under joint ownership of us and NIH pertaining to the use of A3AR agonists for the treatment of uveitis. Such diminution of our proprietary position could have a material adverse effect on our business, results of operation and financial condition.
We license from Leiden University intellectual property, which protects certain small molecules which target the A3AR, in furtherance of our platform technology, and we could lose our rights to this license if a dispute with Leiden University arises or if we fail to comply with the financial and other terms of the license.
We have licensed intellectual property from Leiden University pursuant to a license agreement. The license agreement imposes certain payment, reporting, confidentiality and other obligations on us. In the event that we were to breach any of the obligations and fail to cure, Leiden University would have the right to terminate the license agreement. In addition, Leiden University has the right to terminate the license agreement upon our bankruptcy, insolvency, or receivership. If any dispute arises with respect to our arrangements with Leiden University, such dispute may disrupt our operations and may have a material adverse impact on us if resolved in a manner that is unfavorable to us.
The failure to obtain or maintain patents, licensing agreements, including our current licensing agreements, and other intellectual property could impact our ability to compete effectively.
To compete effectively, we need to develop and maintain a proprietary position with regard to our own technologies, intellectual property, licensing agreements, product candidates and business. Legal standards relating to the validity and scope of claims in the biotechnology and biopharmaceutical fields are still evolving. Therefore, the degree of future protection for our proprietary rights in our core technologies and any products that might be made using these technologies is also uncertain. The risks and uncertainties that we face with respect to our patents and other proprietary rights include the following:
| ● | while the patents we license have been issued, the pending patent applications we have filed may not result in issued patents or may take longer than we expect to result in issued patents; |
| ● | we may be subject to interference proceedings; |
| ● | we may be subject to opposition proceedings in foreign countries; |
| ● | any patents that are issued may not provide meaningful protection; |
| ● | we may not be able to develop additional proprietary technologies that are patentable; |
| ● | other companies may challenge patents licensed or issued to us or our customers; |
| ● | other companies may independently develop similar or alternative technologies, or duplicate our technologies; |
| ● | other companies may design around technologies we have licensed or developed; and |
| ● | enforcement of patents is complex, uncertain and expensive. |
14
If patent rights covering our products and methods are not sufficiently broad, they may not provide us with any protection against competitors with similar products and technologies. Furthermore, if the United States Patent and Trademark Office, or the USPTO, or foreign patent officers issue patents to us or our licensors, others may challenge the patents or design around the patents, or the patent office or the courts may invalidate the patents. Thus, any patents we own or license from or to third parties may not provide any protection against our competitors.
We cannot be certain that patents will be issued as a result of any pending applications, and we cannot be certain that any of our issued patents will give us adequate protection from competing products. For example, issued patents, including the patents licensed by us, may be circumvented or challenged, declared invalid or unenforceable, or narrowed in scope. In addition, since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that we were the first to make our inventions or to file patent applications covering those inventions.
It is also possible that others may obtain issued patents that could prevent us from commercializing our products or require us to obtain licenses requiring the payment of significant fees or royalties in order to enable us to conduct our business. As to those patents that we have licensed, our rights depend on maintaining our obligations to the licensor under the applicable license agreement, and we may be unable to do so.
In addition to patents and patent applications, we depend upon trade secrets and proprietary know-how to protect our proprietary technology. We require our employees, consultants, advisors and collaborators to enter into confidentiality agreements that prohibit the disclosure of confidential information to any other parties. We require our employees and consultants to disclose and assign to us their ideas, developments, discoveries and inventions. These agreements may not, however, provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure.
Costly litigation may be necessary to protect our intellectual property rights and we may be subject to claims alleging the violation of the intellectual property rights of others.
We may face significant expense and liability as a result of litigation or other proceedings relating to patents and other intellectual property rights of others. In the event that another party has also filed a patent application or been issued a patent relating to an invention or technology claimed by us in pending applications, we may be required to participate in an interference proceeding declared by the USPTO to determine priority of invention, which could result in substantial uncertainties and costs for us, even if the eventual outcome were favorable to us. We, or our licensors, also could be required to participate in interference proceedings involving issued patents and pending applications of another entity. An adverse outcome in an interference proceeding could require us to cease using the technology or to license rights from prevailing third parties.
The cost to us of any patent litigation or other proceeding relating to our licensed patents or patent applications, even if resolved in our favor, could be substantial. Our ability to enforce our patent protection could be limited by our financial resources, and may be subject to lengthy delays. If we are unable to effectively enforce our proprietary rights, or if we are found to infringe the rights of others, we may be in breach of our License Agreement.
A third party may claim that we are using inventions claimed by their patents and may go to court to stop us from engaging in our normal operations and activities, such as research, development and the sale of any future products. Such lawsuits are expensive and would consume time and other resources. There is a risk that the court will decide that we are infringing the third party’s patents and will order us to stop the activities claimed by the patents, redesign our products or processes to avoid infringement or obtain licenses (which may not be available on commercially reasonable terms). In addition, there is a risk that a court will order us to pay the other party damages for having infringed their patents.
Moreover, there is no guarantee that any prevailing patent owner would offer us a license so that we could continue to engage in activities claimed by the patent, or that such a license, if made available to us, could be acquired on commercially acceptable terms. In addition, third parties may, in the future, assert other intellectual property infringement claims against us with respect to our product candidates, technologies or other matters.
15
We rely on confidentiality agreements that could be breached and may be difficult to enforce, which could result in third parties using our intellectual property to compete against us.
Although we believe that we take reasonable steps to protect our intellectual property, including the use of agreements relating to the non-disclosure of confidential information to third parties, as well as agreements that purport to require the disclosure and assignment to us of the rights to the ideas, developments, discoveries and inventions of our employees and consultants while we employ them, the agreements can be difficult and costly to enforce. Although we seek to obtain these types of agreements from our contractors, consultants, advisors and research collaborators, to the extent that employees and consultants utilize or independently develop intellectual property in connection with any of our projects, disputes may arise as to the intellectual property rights associated with our products. If a dispute arises, a court may determine that the right belongs to a third party. In addition, enforcement of our rights can be costly and unpredictable. We also rely on trade secrets and proprietary know-how that we seek to protect in part by confidentiality agreements with our employees, contractors, consultants, advisors or others. Despite the protective measures we employ, we still face the risk that:
| ● | these agreements may be breached; |
| ● | these agreements may not provide adequate remedies for the applicable type of breach; |
| ● | our trade secrets or proprietary know-how will otherwise become known; or |
| ● | our competitors will independently develop similar technology or proprietary information. |
International patent protection is particularly uncertain, and if we are involved in opposition proceedings in foreign countries, we may have to expend substantial sums and management resources.
Patent law outside the United States is different than in the United States. Further, the laws of some foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States, if at all. A failure to obtain sufficient intellectual property protection in any foreign country could materially and adversely affect our business, results of operations and future prospects. Moreover, we may participate in opposition proceedings to determine the validity of our foreign patents or our competitors’ foreign patents, which could result in substantial costs and divert management’s resources and attention.
Although most jurisdictions in which we have applied for, intend to apply for, or have been issued patents have patent protection laws similar to those of the United States, some of them do not. For example, we expect to do business in Brazil and India in the future. However, the Brazilian drug regulatory agency, ENVISA, has the authority to nullify patents on the basis of its perceived public interest and the Indian patent law does not allow patent protection for new uses of pharmaceuticals (many of our current patent applications are of such nature). Additionally, due to uncertainty in patent protection law, we have not filed applications in many countries where significant markets exist, including Indonesia, Pakistan, Russia, African countries and Taiwan.
We may be unable to protect the intellectual property rights of the third parties from whom we license certain of our intellectual property or with whom we have entered into other strategic relationships.
Certain of our intellectual property rights are currently licensed from Leiden University, and, in the future, we intend to continue to license intellectual property from Leiden University and/or other universities and/or strategic partners. Such third parties may determine not to protect the intellectual property rights that we license from them and we may be unable to defend such intellectual property rights on our own or we may have to undertake costly litigation to defend the intellectual property rights of such third parties. There can be no assurances that we will continue to have proprietary rights to any of the intellectual property that we license from such third parties or otherwise have the right to use through similar strategic relationships. Any loss or limitations on use with respect to our right to use such intellectual property licensed from third parties or otherwise obtained from third parties with whom we have entered into strategic relationships could have a material adverse effect on our business, results of operations and financial condition.
16
Under applicable U.S. and Israeli law, we may not be able to enforce covenants not to compete and therefore, may be unable to prevent our competitors from benefiting from the expertise of some of our former employees. In addition, employees may be entitled to seek compensation for their inventions irrespective of their agreements with us, which in turn could impact our future profitability.
We generally enter into non-competition agreements with our employees and certain key consultants, or our employment and consulting agreements contain non-competition provisions. These agreements, to the extent they are in place and in effect, prohibit our employees and certain key consultants, if they cease working for us, from competing directly with us or working for our competitors or clients for a limited period of time. We may be unable to enforce these agreements under the laws of the jurisdictions in which our employees work and it may be difficult for us to restrict our competitors from benefitting from the expertise our former employees or consultants developed while working for us. For example, Israeli courts have required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company’s confidential commercial information or the protection of its intellectual property. If we cannot demonstrate that such interests will be harmed, we may be unable to prevent our competitors from benefiting from the expertise of our former employees or consultants and our ability to remain competitive may be diminished.
In addition, Chapter 8 to the Israeli Patents Law, 5727-1967, or the Patents Law, deals with inventions made in the course of an employee’s service and during his or her term of employment, whether or not the invention is patentable, or service inventions. Section 134 of the Patents Law provides that if there is no agreement that explicitly determines whether the employee is entitled to compensation for the service inventions and the extent and terms of such compensation, such determination will be made by the Compensation and Rewards Committee, a statutory committee of the Israeli Patents Office. Although our employees have agreed to assign to us service invention rights, we may face claims demanding remuneration in consideration for assigned inventions. As a consequence of such claims, we could be required to pay additional remuneration or royalties to our current and/or former employees, or be forced to litigate such claims, which could negatively affect our business.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
| ● | Others may be able to make compounds that are the same as or similar to our product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed; |
| ● | We or our licensors or any future strategic partners might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed; |
| ● | We or our licensors or any future strategic partners might not have been the first to file patent applications covering certain of our inventions; |
| ● | Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
| ● | It is possible that our pending patent applications will not lead to issued patents; |
| ● | Issued patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors; |
| ● | Our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; and |
| ● | We may not develop additional proprietary technologies that are patentable. |
17
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an interest in our patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Risks Related to Our Industry
We are subject to government regulations and we may experience delays in obtaining required regulatory approvals in the United States to market our proposed product candidates.
Various aspects of our operations are subject to federal, state or local laws, and rules and regulations, any of which may change from time to time. Costs arising out of any regulatory developments could be time-consuming and expensive and could divert management resources and attention and, consequently, could adversely affect our business operations and financial performance.
Delays in regulatory approval, limitations in regulatory approval and withdrawals of regulatory approval may have a material adverse effect on us. If we experience significant delays in testing or receiving approvals or sign-offs to conduct clinical trials, our product development costs, or our ability to license product candidates, will increase. If the FDA grants regulatory approval to market a product, this approval will be limited to those disease states and conditions for which the product has demonstrated, through clinical trials, to be safe and effective. Any product approvals that we receive in the future could also include significant restrictions on the use or marketing of our products. Product approvals, if granted, can be withdrawn for failure to comply with regulatory requirements or upon the occurrence of adverse events following commercial introduction of the products. Failure to comply with applicable FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our product candidates or us. If approval is withdrawn for a product, or if a product were seized or recalled, we would be unable to sell or license that product and our revenues would suffer. In addition, outside the United States, our ability to market any of our potential products is contingent upon receiving market application authorizations from the appropriate regulatory authorities and these foreign regulatory approval processes include all of the risks associated with the FDA approval process described above.
We expect the healthcare industry to face increased limitations on reimbursement as a result of healthcare reform, which could adversely affect third-party coverage of our products and how much or under what circumstances healthcare providers will prescribe or administer our products.
In both the United States and other countries, sales of our products will depend in part upon the availability of reimbursement from third-party payors, which include governmental authorities, managed-care organizations and other private-health insurers. Third-party payors are increasingly challenging the price and examining the cost effectiveness of medical products and services.
Increasing expenditures for healthcare have been the subject of considerable public attention in the United States. Both private and government entities are seeking ways to reduce or contain healthcare costs. Numerous proposals that would effect changes in the U.S. healthcare system have been introduced or proposed in Congress and in some state legislatures, including reducing reimbursement for prescription products and reducing the levels at which consumers and healthcare providers are reimbursed for purchases of pharmaceutical products.
18
In 2010, the U.S. Congress enacted the Patient Protection and Affordable Care Act of 2010, or the Affordable Care Act. The Affordable Care Act seeks to reduce the federal deficit and the rate of growth in healthcare spending through, among other things, stronger prevention and wellness measures, increased access to primary care, changes in healthcare delivery systems and the creation of health insurance exchanges. Enrollment in the health insurance exchanges began in October 2013. The Affordable Care Act requires the pharmaceutical industry to share in the costs of reform, by, among other things, increasing Medicaid rebates and expanding Medicaid rebates to cover Medicaid managed-care programs. Other components of healthcare reform include funding of pharmaceutical costs for Medicare patients in excess of the prescription drug coverage limit and below the catastrophic coverage threshold. Under the Affordable Care Act, pharmaceutical companies are now obligated to fund 50% of the patient obligation for branded prescription pharmaceuticals in this gap, or “donut hole.”
There have been judicial and congressional challenges to the Affordable Care Act, as well as efforts by the Trump Administration to repeal or replace certain aspects of the Affordable Care Act. Since January 2017, President Trump has signed two Executive Orders and other directives designed to delay the implementation of certain provisions of the Affordable Care Act or otherwise circumvent some of the requirements for health insurance mandated by the Affordable Care Act. However, to date, the Executive Orders have had limited effect and the Congressional activities have not resulted in the passage of a law. If a law is enacted, many if not all of the provisions of the Affordable Care Act may no longer apply to prescription drugs. While we are unable to predict what changes may ultimately be enacted, to the extent that future changes affect how any future products are paid for and reimbursed by government and private payers, our business could be adversely impacted. On December 14, 2018, a federal district court in Texas ruled that the Affordable Care Act is unconstitutional as a result of the Tax Cuts and Jobs Act and the federal income tax reform legislation previously passed by Congress and signed by President Trump on December 22, 2017, that eliminated the individual mandate portion of the Affordable Care Act. The case, Texas, et al, v. United States of America, et al., (N.D. Texas), is an outlier, and the ruling has been stayed by the ruling judge. We are not able to state with any certainty what will be the impact of this court decision on our business pending further court action and possible appeals.
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. In August 2011, President Obama signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of an amount greater than $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to healthcare providers of up to 2.0% per fiscal year, starting in 2013. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several categories of healthcare providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. If we ever obtain regulatory approval and commercialization of our products, these laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our customers and accordingly, our financial operations. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our products may be. Further, the Deficit Reduction Act of 2010, directed CMS to contract a vendor to determine “retail survey prices for covered outpatient drugs that represent a nationwide average of consumer purchase prices for such drugs, net of all discounts and rebates (to the extent any information with respect to such discounts and rebates is available).” This survey information can be used to determine the National Average Drug Acquisition Cost, or NADAC. Some states have indicated that they will reimburse based on the NADAC and this can result in further reductions in the prices paid for various outpatient drugs.
In the fourth quarter of 2018, the Trump Administration announced initiatives that it asserted are intended to result in purportedly lower drug prices. The first initiative, announced on October 15, 2018, involved the plan to a new federal regulation that would require pharmaceutical manufacturers to disclose the list prices of their respective prescription drugs in their television advertisements for their products if the list price is greater than $35. With respect to the second initiative, on October 25, 2018, the Centers for Medicaid and Medicare Services gave Advance Notice of Proposed Rulemaking to propose the implementation of an “International Pricing Index” model for Medicare Part B drugs and biologicals (single source drugs, biologicals and biosimilars). Public comments were due on December 31, 2018 with a proposed rule theoretically being offered as early as Spring 2019 with target implementation of a 5 year pilot program beginning in Spring 2020. While these initiatives have not been put into effect, we are not in a position to know at this time whether they will ever become law or what impact the enactment either of these proposals would have on our business.
19
In February 2019, the Department of Health and Human Services, or HHS, has proposed a regulation that would significantly restrict the availability of certain regulatory safe harbors under the federal Anti-Kickback Statute that are used to facilitate certain types of transactions between manufacturers and pharmacy benefits managers that play a significant role in the pharmaceutical distribution chain. These changes to the Discount Safe Harbors available under the Anti-Kickback Statute would reduce some of the protections currently available to manufacturers that pay negotiated rebates to pharmacy benefits managers, or PBMs, in exchange for these PBMs agreeing to include drugs and biologics on the formularies of the PBM’s downstream customers, primarily the health plans that insure patients for both private commercial plans and government-sponsored plans. While we do not know whether the Trump Administration will be successful in implementing this proposed regulation, its successful implementation could have an impact on both our commercial supply arrangements with health plans and our supply arrangements to health plans that serve beneficiaries of federal health care programs such as Medicare Part D.
As part of its reform of the 340B discount drug program, on October 31, 2018, the Health Resources and Services Administration, or HRSA, at the HHS, issued a notice of proposed rulemaking to move up the effective date of a final rule that would give HHS authority to impose Civil Monetary Penalties on pharmaceutical manufacturers who knowingly and intentionally charged a covered entity more than the statutorily allowed ceiling price for a covered outpatient drug. The final rule is intended to encourage compliance by manufacturers in offering the mandatory 340B ceiling purchase price to eligible purchasers, such as certain qualified health systems or individual hospitals.
Various states, such as California, have also taken steps to consider and enact laws or regulations that are intended to increase the visibility of the pricing of pharmaceutical products with the goal of reducing the prices at which we are able to sell our products. Because these various actual and proposed legislative changes are intended to operate on a state-by-state level rather than a national one, we cannot predict what the full effect of these legislative activities may be on our business in the future.
Although we cannot predict the full effect on our business of the implementation of existing legislation, including the Affordable Care Act or the enactment of additional legislation, we believe that legislation or regulations that reduce reimbursement for or restrict coverage of our products could adversely affect how much or under what circumstances healthcare providers will prescribe or administer our products. This could materially and adversely affect our business by reducing our ability to generate revenue, raise capital, obtain additional collaborators and market our products, following marketing approval. In addition, we believe the increasing emphasis on managed care in the United States has and will continue to put pressure on the price and usage of pharmaceutical products, which may adversely impact any future product sales.
If we or any of our independent contractors, consultants, collaborators, manufacturers, or service providers fail to comply with healthcare and data privacy laws and regulations, we or they could be subject to enforcement actions, which could result in penalties and affect our ability to develop, market and sell our product candidates and may harm our reputation.
We are or may in the future be subject to federal, state, and foreign healthcare and data privacy laws and regulations pertaining to, among other things, fraud and abuse of patients’ rights. These laws and regulations include:
| ● | The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully soliciting, offering, receiving, or paying any remuneration, directly or indirectly, in cash or in kind, to induce or reward purchasing, ordering or arranging for or recommending the purchase or order of any item or service for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid. Liability may be established without a person or entity having actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it. This statute has been interpreted to apply broadly to arrangements between pharmaceutical manufacturers on the one hand and prescribers, patients, purchasers and formulary managers on the other. In addition, the Affordable Care Act amended the Social Security Act to provide that the U.S. government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act, or the FCA. A conviction for violation of the Anti-Kickback Statute requires mandatory exclusion from participation in federal healthcare programs. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and those activities may be subject to scrutiny or penalty if they do not qualify for an exemption or safe harbor; |
20
| ● | The FCA prohibits, among other things, knowingly presenting, or causing to be presented claims for payment of government funds that are false or fraudulent, or knowingly making, using or causing to be made or used a false record or statement material to such a false or fraudulent claim, or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. This statute also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the FCA and to share in any monetary recovery. The FCA prohibits anyone from knowingly presenting, conspiring to present, making a false statement in order to present, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services, including drugs, that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. This law also prohibits anyone from knowingly underpaying an obligation owed to a federal program. Increasingly, U.S. federal agencies are requiring nonmonetary remedial measures, such as corporate integrity agreements in FCA settlements. The U.S. Department of Justice announced in 2016 its intent to follow the “Yates Memo,” taking a far more aggressive approach in pursuing individuals as FCA defendants in addition to the corporations. FCA liability is potentially significant in the healthcare industry because the statute provides for treble damages and mandatory penalties of $5,500 to $11,000 per false claim or statement ($10,781 to $21,563 per false claim or statement for penalties assessed after August 1, 2016 for violations occurring after November 2, 2015, and $10,957 to $21,916 per false claim or statement for penalties assessed after February 3, 2017 for violations occurring after November 2, 2015). Government enforcement agencies and private whistleblowers have investigated pharmaceutical companies for or asserted liability under the FCA for a variety of alleged promotional and marketing activities, such as providing free product to customers with the expectation that the customers would bill federal programs for the product; providing consulting fees and other benefits to physicians to induce them to prescribe products; engaging in promotion for “off-label” uses; and submitting inflated best price information to the Medicaid Rebate Program; |
| ● | The federal False Statements Statute prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry, in connection with the delivery of or payment for healthcare benefits, items, or services; |
| ● | The federal Civil Monetary Penalties Law authorizes the imposition of substantial civil monetary penalties against an entity, such as a pharmaceutical manufacturer, that engages in activities including, among others (1) knowingly presenting, or causing to be presented, a claim for services not provided as claimed or that is otherwise false or fraudulent in any way; (2) arranging for or contracting with an individual or entity that is excluded from participation in federal healthcare programs to provide items or services reimbursable by a federal healthcare program; (3) violations of the federal Anti-Kickback Statute; or (4) failing to report and return a known overpayment; |
| ● | The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of, or payment for, healthcare benefits, items or services; similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
21
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, which imposes requirements on certain types of people and entities relating to the privacy, security, and transmission of individually identifiable health information, and requires notification to affected individuals and regulatory authorities of certain breaches of security of individually identifiable health information; |
| ● | The federal Physician Payment Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, to report annually to the Centers for Medicare & Medicaid Services information related to payments and other transfers of value to physicians, other healthcare providers and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members, which is published in a searchable form on an annual basis; |
| ● | State laws comparable to each of the above federal laws, such as, for example, anti-kickback and false claims laws that may be broader in scope and also apply to commercial insurers and other non-federal; |
| ● | Payors requirements for mandatory corporate regulatory compliance programs, and laws relating to patient data privacy and security. Other state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts; and |
| ● | In the European Union, the General Data Protection Regulation, or the GDPR—Regulation EU 2016/679—which was adopted in May 2016 and has become applicable on May 25, 2018. The GDPR is further intended to harmonize data protection requirements across the European Union member states by establishing new and expanded operational requirements for entities that collect, process or use personal data generated in the European Union, including consent requirements for disclosing the way personal information will be used, information retention requirements, and notification requirements in the event of a data breach. |
If our operations are found to be in violation of any such healthcare laws and regulations, we may be subject to penalties, including administrative, civil and criminal penalties, monetary damages, disgorgement, imprisonment, the curtailment or restructuring of our operations, loss of eligibility to obtain approvals from the FDA or foreign regulatory authorities, or exclusion from participation in government contracting, healthcare reimbursement or other government programs, including Medicare and Medicaid, any of which could adversely our financial results. Any action against us for an alleged or suspected violation could cause us to incur significant legal expenses and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with applicable laws and regulations may be costly to us in terms of money, time and resources.
Our employees, principal investigators, consultants, commercial partners or vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards.
We are also exposed to the risk of employees, independent contractors, principal investigators, consultants, commercial partners or vendors engaging in fraud or other misconduct. Misconduct by employees, independent contractors, principal investigators, consultants, commercial partners and vendors could include intentional failures to comply with EU regulations, to provide accurate information to the EMA or EU Member States authorities or to comply with manufacturing or quality standards we have or will have established. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices such as promotion of products by medical practitioners. The EU Member States in which we operate have different statutory provisions regulating the cooperation of pharmaceutical companies with healthcare professionals. In addition to these statutory provisions, codes of conduct issued by business associations or other non-statutory standards may be applicable to our activities. Both statutory provisions and non-statutory codes or standards restrict payments or other benefits provided to healthcare professionals, and in case of non-compliance, may result in severe sanctions such as bans, administrative fines, criminal fines or even imprisonment. The advertising of medicinal products for human use in the EU is regulated by Title VIII of European Directive 2001/83/EC. These provisions have been implemented into the law of the EU member States. Such laws inter alia restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and serious and irreparable harm to our reputation.
22
This could also apply with respect to data privacy. In the EU, the EU Directive 95/46/EEC was replaced by the GDPR on May 25, 2018. The GDPR as an EU regulation does not have to be implemented into Member States’ national law, but applies directly in all Member States since May 25, 2018. It applies to companies with an establishment in the European Economic Area (EEA) and to certain other companies not in the EEA that offer or provide goods or services to individuals located in the EEA or monitor individuals located in the EEA. The GDPR implements more stringent operational requirements for controllers of personal data, including, for example, expanded disclosures about how personal information is to be used, limitations on retention of information, increased requirements pertaining to health data and pseudonymized (i.e., key-coded) data, increased cyber security requirements, mandatory data breach notification requirements and higher standards for controllers to demonstrate that they have obtained a valid legal basis for certain data processing activities. The GDPR provides that EU Member States may continue to make their own further laws and regulations in relation to the processing of genetic, biometric or health data, which could result in continued or new differences between Member States, limit our ability to use and share personal data or could cause our costs to increase, and harm our business and financial condition. We are also subject to evolving and strict rules on the transfer of personal data out of the European Union to the United States. Further prospective revision of the Directive on privacy and electronic communications (Directive 2002/58/EC), or ePrivacy Directive, may affect our marketing communications.
We are in the process of implementing policies and procedures to ensure compliance with the GDPR and its requirements. Our actual or alleged failure to comply with this regulation, or to protect personal data, could result in enforcement actions and significant penalties against us, which could result in negative publicity, increase our operating costs, subject us to claims or other remedies and have a material adverse effect on our business, financial condition, and results of operations. It is not always possible to identify and deter misconduct by employees or other parties. The precautions we take to detect and prevent such activity may not protect us from legal or regulatory action resulting from a failure to comply with applicable laws or regulations. Misconduct by our employees, principal investigators, consultants, commercial partners or vendors could result in significant financial penalties, criminal sanctions, civil law claims and/or negative media coverage, and thus have a material adverse effect on our business, including through the imposition of significant fines or other sanctions, and our reputation. In particular, failure to comply with EU laws, including failure under the GDPR, ePrivacy Directive and other laws relating to the security of personal data may result in fines up to €20,000,000 or up to 4% of the total worldwide annual turnover of the preceding financial year, if greater, and other administrative penalties including criminal liability, which may be onerous and adversely affect our business, financial condition, results of operations and prospects. Failure to comply with the GDPR and related laws may also give risk to increase risk of private actions, including a new form of class action that is available under the GDPR.
23
Risks Related to Our Ordinary Shares and ADSs
There can be no assurance that we will not be a passive foreign investment company, or PFIC, for U.S. federal income tax purposes in 2019 or in any subsequent year. There may be negative tax consequences for U.S. taxpayers that are holders of our ordinary shares or our ADSs.
We will be treated as a PFIC for U.S. federal income tax purposes in any taxable year in which either (i) at least 75% of our gross income is “passive income” or (ii) on average at least 50% of our assets by value produce passive income or are held for the production of passive income. Passive income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in a public offering. In determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account. If we were to be characterized as a PFIC for U.S. federal income tax purposes in any taxable year during which a U.S. shareholder owns our ordinary shares or ADSs, and such U.S. shareholder does not make an election to treat us as a “qualified electing fund,” or QEF, or make a “mark-to-market” election, then “excess distributions” to such U.S. shareholder, and any gain realized on the sale or other disposition of our ordinary shares or ADSs will be subject to special rules. Under these rules: (i) the excess distribution or gain would be allocated ratably over the U.S. shareholder’s holding period for the ordinary shares (or ADSs, as the case may be); (ii) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (iii) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, if the U.S. Internal Revenue Service determines that we are a PFIC for a year with respect to which we have determined that we were not a PFIC, it may be too late for a U.S. shareholder to make a timely QEF or mark-to-market election. U.S. shareholders who hold our ordinary shares or ADSs during a period when we are a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC in subsequent years, subject to exceptions for U.S. shareholders who made a timely QEF or mark-to-market election. A U.S. shareholder can make a QEF election by completing the relevant portions of and filing IRS Form 8621 in accordance with the instructions thereto. Upon request, we will annually furnish U.S. shareholders with information needed in order to complete IRS Form 8621 (which form would be required to be filed with the IRS on an annual basis by the U.S. shareholder) and to make and maintain a valid QEF election for any year in which we or any of our subsidiaries that we control is a PFIC.
Issuance of additional equity securities may adversely affect the market price of our ADSs or ordinary shares.
We are currently authorized to issue 500,000,000 ordinary shares. As of March 21, 2019, we had 44,875,482 ordinary shares issued and outstanding and we had no preferred shares outstanding. As of March 21, 2019, we also had warrants to purchase 18,035,006 ordinary shares and options to purchase 2,177,400 ordinary shares outstanding, of which options to purchase 880,529 ordinary shares are currently fully vested or vest within the next 60 days.
To the extent that ADSs or ordinary shares are issued or options and warrants are exercised, holders of our ADSs and our ordinary shares will experience dilution. In addition, in the event of any future issuances of equity securities or securities convertible into or exchangeable for ADSs or ordinary shares, holders of our ADSs or our ordinary shares may experience dilution. We also consider from time to time various strategic alternatives that could involve issuances of additional ADSs or ordinary shares, including but not limited to acquisitions and business combinations, but do not currently have any definitive plans to enter into any of these transactions.
We have no plans to pay dividends on our ordinary shares, and you may not receive funds without selling our ADSs or ordinary shares.
We have not declared or paid any cash dividends on our ordinary shares, nor do we expect to pay any cash dividends on our ordinary shares for the foreseeable future. We currently intend to retain any additional future earnings to finance our operations and growth and for future stock repurchases and, therefore, we have no plans to pay cash dividends on our ordinary shares at this time. Any future determination to pay cash dividends on our ordinary shares will be at the discretion of our board of directors and will be dependent on our earnings, financial condition, operating results, capital requirements, any contractual restrictions, and other factors that our board of directors deems relevant. Accordingly, you may have to sell some or all of our ADSs or ordinary shares in order to generate cash from your investment. You may not receive a gain on your investment when you sell our ADSs or ordinary shares and may lose the entire amount of your investment.
24
The market price of our ordinary shares and ADSs is subject to fluctuation, which could result in substantial losses by our investors.
The stock market in general and the market price of our ordinary shares on the TASE and our ADSs on the NYSE American is subject to fluctuation, and changes in our share price may be unrelated to our operating performance. The market price of our ordinary shares and ADSs are and will be subject to a number of factors, including:
| ● | announcements of technological innovations or new products by us or others; | |
| ● | announcements by us of significant strategic partnerships, out-licensing, in-licensing, joint ventures, acquisitions or capital commitments; | |
| ● | expiration or terminations of licenses, research contracts or other collaboration agreements; | |
| ● | public concern as to the safety of drugs we, our licensees or others develop; | |
| ● | general market conditions; | |
| ● | the volatility of market prices for shares of biotechnology companies generally; | |
| ● | success of research and development projects; | |
| ● | success in clinical and preclinical studies; | |
| ● | departure of key personnel; | |
| ● | developments concerning intellectual property rights or regulatory approvals; | |
| ● | variations in our and our competitors’ results of operations; | |
| ● | changes in earnings estimates or recommendations by securities analysts, if our ordinary shares or ADSs are covered by analysts; | |
| ● | changes in government regulations or patent decisions; | |
| ● | developments by our licensees; and | |
| ● | general market conditions and other factors, including factors unrelated to our operating performance. |
These factors and any corresponding price fluctuations may materially and adversely affect the market price of our ordinary shares and our ADSs and result in substantial losses by our investors.
Additionally, market prices for securities of biotechnology and pharmaceutical companies historically have been very volatile. The market for these securities has from time to time experienced significant price and volume fluctuations for reasons unrelated to the operating performance of any one company. In the past, following periods of market volatility, shareholders have often instituted securities class action litigation and we have been named in the past in a lawsuit requesting recognition as a class action, in which we ultimately prevailed. If we were involved in securities litigation, it could have a substantial cost and divert resources and attention of management from our business, even if we are successful.
Future sales of our ordinary shares or ADSs could reduce the market price of our ordinary shares and ADSs.
25
Substantial sales of our ordinary shares or our ADSs either on the TASE or on the NYSE American, as applicable, may cause the market price of our ordinary shares or our ADSs to decline.
Sales by us or our security-holders of substantial amounts of our ordinary shares or our ADSs, or the perception that these sales may occur in the future, could cause a reduction in the market price of our ordinary shares or our ADSs. The issuance of any additional ordinary shares or ADSs, or any securities that are exercisable for or convertible into our ordinary shares or our ADSs, may have an adverse effect on the market price of our ordinary shares or our ADSs, as applicable, and will have a dilutive effect on our shareholders.
We may not satisfy the NYSE American requirements for continued listing. If we cannot satisfy these requirements, the NYSE American could delist our securities.
Our ADSs are listed on the NYSE American under the symbol “CANF.” To continue to be listed on the NYSE American, we are required to satisfy a number of conditions, including maintaining a share price and shareholders’ equity above certain thresholds. If we are delisted from the NYSE American, trading in our securities may be conducted, if available, on the OTC Markets or, if available, via another market. In the event of such delisting, our shareholders would likely find it significantly more difficult to dispose of, or to obtain accurate quotations as to the value of our securities, and our ability to raise future capital through the sale of our securities could be severely limited. In addition, if our securities were delisted from the NYSE American, our ADSs could be considered a “penny stock” under the U.S. federal securities laws. Additional regulatory requirements apply to trading by broker-dealers of penny stocks that could result in the loss of an effective trading market for our securities. Moreover, if our ADSs were delisted from the NYSE American, we will no longer be exempt from certain provisions of the Israeli Securities Law, and therefore will have increased disclosure requirements.
ADS holders are not shareholders and do not have shareholder rights.
The Bank of New York Mellon, as Depositary, delivers our ADSs. Each ADS represents two of our ordinary shares. ADS holders will not be treated as shareholders and do not have the rights of shareholders. The Depositary will be the holder of the shares underlying our ADSs. Holders of ADSs will have ADS holder rights. A deposit agreement among us, the Depositary, ADS holders and the beneficial owners of ADSs sets out ADS holder rights as well as the rights and obligations of the Depositary. New York law governs the deposit agreement and our ADSs. Our shareholders have shareholder rights. Israeli law and our Amended and Restated Articles of Association govern shareholder rights. ADS holders do not have the same voting rights as our shareholders. Shareholders are entitled to our notices of general meetings and to attend and vote at our general meetings of shareholders. At a general meeting, every shareholder present (in person or by proxy, attorney or representative) and entitled to vote has one vote. This is subject to any other rights or restrictions which may be attached to any shares. ADS holders may instruct the Depositary how to vote the number of deposited shares their ADSs represent. Otherwise, you won’t be able to exercise your right to vote unless you withdraw the shares. However, you may not know about the meeting enough in advance to withdraw the shares. The Depositary will notify ADS holders of shareholders’ meetings and arrange to deliver our voting materials to them if we ask it to. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the Depositary how to vote. For instructions to be valid, they must reach the Depositary by a date set by the Depositary. The Depositary will try, as far as practical, subject to the laws of Israel and our Amended and Restated Articles of Association or similar documents, to vote or to have its agents vote the shares or other deposited securities as instructed by ADS holders. The Depositary will only vote or attempt to vote as instructed. We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the Depositary to vote your shares. In addition, the Depositary and its agents are not responsible for failing to carry out voting instructions or for the matter of carrying out voting instructions. This means that you may not be able to exercise your right to vote and there may be nothing you can do if your shares are not voted as requested.
ADS holders do not have the same rights to receive dividends or other distributions as our shareholders. Subject to any special rights or restrictions attached to a share, the directors may determine that a dividend will be payable on a share and fix the amount, the time for payment and the method for payment (although we have never declared or paid any cash dividends on our ordinary shares and we do not anticipate paying any cash dividends in the foreseeable future). Dividends and other distributions payable to our shareholders with respect to our ordinary shares generally will be payable directly to them. Any dividends or distributions payable with respect to ordinary shares deposited in the ADS facility will be paid to the Depositary, which has agreed to pay to ADS holders the cash dividends or other distributions it or the custodian receives on shares or other deposited securities, after deducting its fees and expenses. ADS holders will receive these distributions in proportion to the number of ordinary shares their ADSs represent. In addition, there may be certain circumstances in which the Depositary may not pay ADS holder’s amounts distributed by us as a dividend or distribution.
26
Our ordinary shares and our ADSs are traded on different markets and this may result in price variations.
Our ordinary shares have traded on the TASE since October 2005 and our ADSs have been listed on the NYSE American since November 2013. Trading on these markets will take place in different currencies (U.S. dollars on the NYSE American and NIS on the TASE), and at different times (resulting from different time zones, different trading days and different public holidays in the United States and Israel). The trading prices of our securities on these two markets may differ due to these and other factors. Any decrease in the price of our securities on one of these markets could cause a decrease in the trading price of our securities on the other market.
We have incurred significant additional increased costs as a result of the listing of our ADSs for trading on the NYSE American, and our management is required to devote substantial time to new compliance initiatives as well as to compliance with ongoing U.S. and Israeli reporting requirements.
As a public company in the United States, we incur additional significant accounting, legal and other expenses that we did not incur before becoming a reporting company in the United States. We also incur costs associated with corporate governance requirements of the SEC and the NYSE American Company Guide, as well as requirements under Section 404 and other provisions of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act as a result of our ADSs being listed on the NYSE American. These rules and regulations have increased our legal and financial compliance costs, introduced new costs such as investor relations, stock exchange listing fees and shareholder reporting, and made some activities more time consuming and costly. The implementation and testing of such processes and systems may require us to hire outside consultants and incur other significant costs. Any future changes in the laws and regulations affecting public companies in the United States and Israel, including Section 404 and other provisions of the Sarbanes-Oxley Act, the rules and regulations adopted by the SEC and the NYSE American Company Guide, as well as applicable Israeli reporting requirements, for so long as they apply to us, may result in increased costs to us as we respond to such changes. These laws, rules and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors, our board committees or as executive officers.
As a foreign private issuer, we are permitted to follow certain home country corporate governance practices instead of applicable SEC and NYSE American requirements, which may result in less protection than is accorded to investors under rules applicable to domestic issuers.
As a foreign private issuer, we will be permitted to follow certain home country corporate governance practices instead of those otherwise required under the NYSE American Company Guide for domestic issuers. For instance, we may follow home country practice in Israel with regard to, among other things, composition and function of the audit committee and other committees of our Board of Directors and certain general corporate governance matters. In addition, in certain instances we will follow our home country law, instead of the NYSE American Company Guide, which requires that we obtain shareholder approval for certain dilutive events, such as an issuance that will result in a change of control of the company, certain transactions other than a public offering involving issuances of a 20% or more interest in the company and certain acquisitions of the stock or assets of another company. We comply with the director independence requirements of the NYSE American Company Guide, including the requirement that a majority of the Board of Directors be independent. Following our home country governance practices as opposed to the requirements that would otherwise apply to a U.S. company listed on the NYSE American may provide less protection than is accorded to investors under the NYSE American Company Guide applicable to domestic issuers.
In addition, as a foreign private issuer, we are exempt from the rules and regulations under the U.S. Securities Exchange Act of 1934, as amended, or the Exchange Act, related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as domestic companies whose securities are registered under the Exchange Act.
27
Because we became a reporting company under the Exchange Act by means of filing a Form 20-F, we may have difficulty attracting the attention of research analysts at major brokerage firms.
Because we did not become a reporting company by conducting an underwritten initial public offering in the United States, we may have difficulty attracting the attention of security analysts at major brokerage firms in order for them to provide coverage of our company. The failure to receive research coverage or support in the market for our shares will have an adverse effect on our ability to develop a liquid market for our ADSs.
If we are unable to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act as they apply to a foreign private issuer that is listed on a U.S. exchange, or our internal control over financial reporting is not effective, the reliability of our financial statements may be questioned and our share price and the ADS price may suffer.
We are subject to the requirements of the Sarbanes-Oxley Act. Section 404 of the Sarbanes-Oxley Act requires companies subject to the reporting requirements of the U.S. securities laws to do a comprehensive evaluation of its and its subsidiaries’ internal control over financial reporting. To comply with this statute, we must document and test our internal control procedures and our management and issue a report concerning our internal control over financial reporting. In addition, under the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, emerging growth companies, like ourselves, are exempt from certain reporting requirements, including the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act. Under this exemption, our auditor will not be required to attest to and report on our management’s assessment of our internal control over financial reporting until the date we are no longer an emerging growth company as defined in the JOBS Act, because we are taking advantage of the exemptions contained in the JOBS Act. We will need to prepare for compliance with Section 404 by strengthening, assessing and testing our system of internal controls to provide the basis for our report. However, the continuous process of strengthening our internal controls and complying with Section 404 is complicated and time-consuming. Furthermore, as our business continues to grow both domestically and internationally, our internal controls will become more complex and will require significantly more resources and attention to ensure our internal controls remain effective overall. During the course of the testing, our management may identify material weaknesses or significant deficiencies, which may not be remedied in a timely manner to meet the deadline imposed by the Sarbanes-Oxley Act. If our management cannot favorably assess the effectiveness of our internal control over financial reporting, or our independent registered public accounting firm identifies material weaknesses in our internal controls, investor confidence in our financial results may weaken, and the market price of our securities may suffer.
While we currently qualify as an “emerging growth company” under the JOBS Act, we will cease to be an emerging growth company on or before the end of 2019, and at such time our costs and the demands placed upon our management will increase.
As an “emerging growth company” under the JOBS Act, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements. We are an emerging growth company until the earliest of: (i) the last day of the fiscal year during which we had total annual gross revenues of $1.07 billion or more, (ii) the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement (i.e., December 31, 2019), (iii) the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt or (iv) the date on which we are deemed a “large accelerated issuer” as defined in Regulation S-K of the Securities Act. For so long as we remain an emerging growth company, we will not be required to:
| ● | have an auditor report on our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act; |
| ● | comply with any requirement that may be adopted by the Public Company Accounting Oversight Board, or the PCAOB, regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis); |
28
| ● | submit certain executive compensation matters to shareholders advisory votes pursuant to the “say on frequency” and “say on pay” provisions (requiring a non-binding shareholder vote to approve compensation of certain executive officers) and the “say on golden parachute” provisions (requiring a non-binding shareholder vote to approve golden parachute arrangements for certain executive officers in connection with mergers and certain other business combinations) of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010; and |
| ● | include detailed compensation discussion and analysis in our filings under the Exchange Act, and instead may provide a reduced level of disclosure concerning executive compensation. |
We cannot predict if investors will find our ordinary shares or ADSs less attractive because we may rely on these exemptions. If some investors find our ordinary shares less attractive as a result, there may be a less active trading market for our ordinary shares, and our share price may become more volatile and decline.
Risks Related to Our Operations in Israel
We conduct our operations in Israel and therefore our results may be adversely affected by political, economic and military instability in Israel and its region.
Our headquarters, all of our operations and some of our suppliers and third party contractors are located in central Israel and our key employees, officers and most of our directors are residents of Israel. Accordingly, political, economic and military conditions in Israel and the surrounding region may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its Arab neighbors. Any hostilities involving Israel or the interruption or curtailment of trade within Israel or between Israel and its trading partners could adversely affect our operations and results of operations and could make it more difficult for us to raise capital. During the winter of 2008, winter of 2012 and the summer of 2014, Israel was engaged in an armed conflict with Hamas, a militia group and political party operating in the Gaza Strip, and during the summer of 2006, Israel was engaged in an armed conflict with Hezbollah, a Lebanese Islamist Shiite militia group and political party. Israel faces political tension with respect to its relationships with Turkey, Iran and certain Arab neighbor countries. In addition, recent conflicts involved missile strikes against civilian targets in various parts of Israel, and negatively affected business conditions in Israel. Recent political uprisings and social unrest in various countries in the Middle East and North Africa are affecting the political stability of those countries. This instability may lead to deterioration of the political relationships that exist between Israel and these countries, and have raised concerns regarding security in the region and the potential for armed conflict. Any armed conflicts, terrorist activities or political instability in the region could adversely affect business conditions and could harm our results of operations. For example, any major escalation in hostilities in the region could result in a portion of our employees and service providers being called up to perform military duty for an extended period of time. Parties with whom we do business have sometimes declined to travel to Israel during periods of heightened unrest or tension, forcing us to make alternative arrangements when necessary. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements. Any future deterioration in the political and security situation in Israel will negatively impact our business.
Our commercial insurance does not cover losses that may occur as a result of events associated with the security situation in the Middle East. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflicts or political instability in the region would likely negatively affect business conditions and could harm our results of operations.
29
Further, in the past, the State of Israel and Israeli companies have been subjected to an economic boycott. Several countries still restrict business with the State of Israel and with Israeli companies. These restrictive laws and policies may have an adverse impact on our operating results, financial condition or the expansion of our business.
Our operations may be disrupted as a result of the obligation of Israeli citizens to perform military service.
Many Israeli citizens, including Motti Farbstein, our Chief Operating and Financial Officer, are obligated to perform one month, and in some cases more, of annual military reserve duty until they reach the age of 40 (or older, for reservists with certain occupations) and, in the event of a military conflict, may be called to active duty. In response to increases in terrorist activity, there have been periods of significant call-ups of military reservists. It is possible that there will be military reserve duty call-ups in the future. Our operations could be disrupted by such call-ups, which may include the call-up of Motti Farbstein. Such disruption could materially adversely affect our business, financial condition and results of operations.
Because a certain portion of our expenses is incurred in currencies other than U.S. dollars, our results of operations may be harmed by currency fluctuations and inflation.
From our inception through January 1, 2018, our functional and presentation currency was the NIS. Management conducted a review of the functional currency and decided to change its functional and presentation currency to U.S. dollars from the NIS effective January 1, 2018. These changes were based on an assessment by our management that U.S. dollars is the primary currency of the economic environment in which we operate. Part of our expenses are payable in U.S. dollars or in Euros as well as the revenues from our licensing arrangements that are payable in U.S. dollars and Canadian dollars, we expect our revenues from future licensing arrangements to be denominated in U.S. dollars or in Euros. To date, we have not engaged in hedging transactions. Although the Israeli rate of inflation has not had a material adverse effect on our financial condition during 2016, 2017, or 2018 to date, we may, in the future, decide to enter into currency hedging transactions to decrease the risk of financial exposure from fluctuations in the exchange rates of the currencies mentioned above in relation to U.S. dollars. These measures, however, may not adequately protect us from material adverse effects.
Provisions of Israeli law may delay, prevent or otherwise impede a merger with, or an acquisition of, our Company, which could prevent a change of control, even when the terms of such a transaction are favorable to us and our shareholders.
Israeli corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to these types of transactions. For example, a merger may not be consummated unless at least 50 days have passed from the date that a merger proposal was filed by each merging company with the Israel Registrar of Companies and at least 30 days from the date that the shareholders of both merging companies approved the merger. In addition, a majority of each class of securities of the target company must approve a merger. Moreover, a full tender offer can only be completed if the acquirer receives at least 95% of the issued share capital; provided that, pursuant to an amendment to the Companies Law, 5759-1999, as amended, or the Israeli Companies Law, effective as of May 15, 2011, a majority of the offerees that do not have a personal interest in such tender offer shall have approved the tender offer; except that, if the total votes to reject the tender offer represent less than 2% of our issued and outstanding share capital, in the aggregate, approval by a majority of the offerees that do not have a personal interest in such tender offer is not required to complete the tender offer, and the shareholders, including those who indicated their acceptance of the tender offer, may, at any time within six months following the completion of the tender offer, petition the court to alter the consideration for the acquisition (unless the acquirer stipulated in the tender offer that a shareholder that accepts the offer may not seek appraisal rights).
Furthermore, Israeli tax considerations may make potential transactions unappealing to us or to our shareholders whose country of residence does not have a tax treaty with Israel exempting such shareholders from Israeli tax. For example, Israeli tax law does not recognize tax-free share exchanges to the same extent as U.S. tax law. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of numerous conditions, including a holding period of two years from the date of the transaction during which sales and dispositions of shares of the participating companies are restricted. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no actual disposition of the shares has occurred.
30
These and other similar provisions could delay, prevent or impede an acquisition of us or our merger with another company, even if such an acquisition or merger would be beneficial to us or to our shareholders. See “Item 10. Additional Information—B. Memorandum and Articles of Association.”
It may be difficult to enforce a U.S. judgment against us and our officers and directors named in this Annual Report on Form 20-F in Israel or the United States, or to serve process on our officers and directors.
We are incorporated in Israel. All of our executive officers and directors listed in this Annual Report on Form 20-F reside outside of the United States, and all of our assets and most of the assets of our executive officers and directors are located outside of the United States. Therefore, a judgment obtained against us or most of our executive officers and all of our directors in the United States, including one based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not be enforced by an Israeli court. It also may be difficult for you to effect service of process on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel.
Your rights and responsibilities as a shareholder will be governed by Israeli law which may differ in some respects from the rights and responsibilities of shareholders of U.S. companies.
We are incorporated under Israeli law. The rights and responsibilities of the holders of our shareholders are governed by our Amended and Restated Articles of Association and Israeli law. These rights and responsibilities differ in some respects from the rights and responsibilities of shareholders in typical U.S.-based corporations. In particular, a shareholder of an Israeli company has a duty to act in good faith toward the company and other shareholders and to refrain from abusing its power in the company, including, among other things, in voting at the general meeting of shareholders on matters such as amendments to a company’s articles of association, increases in a company’s authorized share capital, mergers and acquisitions and interested party transactions requiring shareholder approval. In addition, a shareholder who knows that it possesses the power to determine the outcome of a shareholder vote or to appoint or prevent the appointment of a director or executive officer in the company has a duty of fairness toward the company. There is limited case law available to assist us in understanding the implications of these provisions that govern shareholders’ actions. These provisions may be interpreted to impose additional obligations and liabilities on holders of our shareholders that are not typically imposed on shareholders of U.S. corporations.
ITEM 4. Information on the Company
A. History and Development of the Company
Our legal name is Can-Fite BioPharma Ltd. and our commercial name is “Can-Fite.” We are a company limited by shares organized under the laws of the State of Israel. Our principal executive offices are located at 10 Bareket Street, Kiryat Matalon, Petah-Tikva 4951778 Israel. Our telephone number is +972 (3) 924-1114.
We were founded on September 11, 1994 by Pnina Fishman, Ph.D., our Chief Executive Officer and a director, and Ilan Cohn, Ph.D., our Chairman of the Board of Directors, under the name Can-Fite Technologies Ltd. On January 7, 2001, we changed our name to Can-Fite BioPharma Ltd. We completed our initial public offering in Israel in October 2005 and our ordinary shares are traded on the TASE under the symbol “CFBI.” On October 2, 2012, our ADSs began trading over the counter in the United States under the symbol “CANFY” and on November 19, 2013, our ADSs began trading on the NYSE American under the symbol “CANF.”
31
In November 2011, through a series of transactions, we spun-off our activity in the ophthalmic field to our now former subsidiary, OphthaliX, a Delaware corporation and successor-in-interest to Denali Concrete Management, Inc., a Nevada corporation, whose common shares were traded in the United States on OTC under the symbol “OPLI.” In the spin-off transactions, we granted an exclusive license for the use of our Piclidenoson drug candidate in the ophthalmic field, or the License Agreement, to Eye-Fite Ltd., an Israel limited company, or Eye-Fite, and transferred our issued and outstanding ordinary shares in Eye-Fite to OphthaliX in exchange for an 86.7% interest in OphthaliX. In connection with the spin-off transactions, OphthaliX completed a series of private placement financing transactions. Following the spin-off transactions and the private placement financing transactions, we held approximately 82% interest in OphthaliX. In July 2016, OphthaliX released top-line results from its Phase II clinical trial of Piclidenoson for the treatment of glaucoma. In this trial, no statistically significant differences were found between the Piclidenoson treated group and the placebo group in the primary endpoint of lowering IOP. High IOP is a characteristic of glaucoma. Piclidenoson was found to have a favorable safety profile and was well tolerated. Based on these overall results, OphthaliX saw no immediate path forward in glaucoma and ceased active business operations. Subsequently, on May 21, 2017, OphthaliX and a wholly owned private Israeli subsidiary of OphthaliX, Bufiduck Ltd, or the Merger Sub, and Wize Pharma Ltd., or Wize Israel, an Israeli company formerly listed on the TASE entered into an Agreement and Plan of Merger, or the Merger Agreement, providing for the merger of the Merger Sub with and into Wize Israel, with Wize Israel becoming a wholly-owned subsidiary of OphthaliX and the surviving corporation of the merger, or the Merger. On November 16, 2017, the Merger was completed. As a result of the Merger, our ownership of OphthaliX, immediately post-Merger, became approximately 8% of the outstanding shares of common stock. In addition, immediately prior to the Merger, OphthaliX sold on an “as is” basis to us all the ordinary shares of Eye-Fite in exchange for the irrevocable cancellation and waiver of all indebtedness owed by OphthaliX and Eye-Fite to us, including approximately $5 million of deferred payments owed by OphthaliX and Eye-Fite to us and, as part of the purchase of Eye-Fite, we also assumed certain accrued milestone payments in the amount of $175,000 under a license agreement previously entered into with NIH. In addition, that certain License Agreement granted to OphthaliX by us and a related services agreement was terminated. See “Item 7. Major Shareholders and Related Party Transactions—B. Related Party Transactions.”
Our capital expenditures for the years ended December 31, 2018, 2017 and 2016 were $33,000, $7,000, and $10,000, respectively. Our current capital expenditures are made solely within Israel and primarily consist of the acquisition of computers and related communications equipment. Such capital expenditures are financed internally.
We qualify as an “emerging growth company,” as defined in the JOBS Act. For as long as we are deemed an emerging growth company, we may take advantage of specified reduced reporting and other regulatory requirements that are generally unavailable to other public companies. These provisions include:
| ● | an exemption from the auditor attestation requirement in the assessment of our internal controls over financial reporting required by Section 404 of the Sarbanes-Oxley Act of 2002; |
| ● | an exemption from compliance with any new requirements adopted by the PCAOB requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about our audit and our financial statements; and |
| ● | reduced disclosure about our executive compensation arrangements. |
We expect to continue to be deemed an emerging growth company until at least December 31, 2019.
We use our website (http://www.canfite.com) as a channel of distribution of Company information. The information we post on our website may be deemed material. Accordingly, investors should monitor the website, in addition to following our press releases, SEC filings and public conference calls and webcasts. The contents of our website are not, however, a part of this Annual Report on Form 20-F.
B. Business Overview
We are a clinical-stage biopharmaceutical company focused on developing orally bioavailable small molecule therapeutic products for the treatment of cancer, liver and inflammatory disease and sexual dysfunction. Our platform technology utilizes the Gi protein associated A3AR as a therapeutic target. A3AR is highly expressed in inflammatory and cancer cells, and not significantly expressed in normal cells, suggesting that the receptor could be a unique target for pharmacological intervention. Our pipeline of drug candidates are synthetic, highly specific agonists and allosteric modulators, or ligands or molecules that initiate molecular events when binding with target proteins, targeting the A3AR.
32
Our product pipeline is based on the research of Dr. Pnina Fishman, who investigated a clinical observation that tumor metastasis can be found in most body tissues, but are rarely found in muscle tissue, which constitutes approximately 60% of human body weight. Dr. Fishman’s research revealed that one reason that striated muscle tissue is resistant to tumor metastasis is that muscle cells release small molecules which bind with high selectivity to the A3AR. As part of her research, Dr. Fishman also discovered that A3ARs have significant expression in tumor and inflammatory cells, whereas normal cells have low or no expression of this receptor. The A3AR agonists and allosteric modulators, currently our pipeline of drug candidates, bind with high selectivity and affinity to the A3ARs and upon binding to the receptor initiate down-stream signal transduction pathways resulting in apoptosis, or programmed cell death, of tumors and inflammatory cells and to the inhibition of inflammatory cytokines. Cytokines are proteins produced by cells that interact with cells of the immune system in order to regulate the body’s response to disease and infection. Overproduction or inappropriate production of certain cytokines by the body can result in disease.
Our product candidates, CF101, CF102 and CF602, are being developed to treat autoimmune inflammatory indications, oncology and liver diseases as well as sexual dysfunction. CF101, also known as Piclidenoson, is in an advanced stage of clinical development for the treatment of autoimmune-inflammatory diseases, including rheumatoid arthritis and psoriasis. CF102, also known as Namodenoson, is being developed for the treatment of HCC and has orphan drug designation for the treatment of HCC in the United States and Europe. Namodenoson was granted Fast Track designation by the FDA as a second line treatment to improve survival for patients with advanced HCC who have previously received Nexavar (sorafenib). Namodenoson is also being developed for the treatment of NASH, following our study which revealed compelling pre-clinical data on Namodenoson in the treatment of NASH, a disease for which no FDA approved therapies currently exist. CF602 is our second generation allosteric drug candidate for the treatment of sexual dysfunction, which has shown efficacy in the treatment of erectile dysfunction in preclinical studies and we are investigating additional compounds, targeting A3AR, for the treatment of sexual dysfunction. Preclinical studies revealed that our drug candidates have potential to treat additional inflammatory diseases, such as Crohn’s disease, oncological diseases and viral diseases, such as the JC virus, and obesity.
We believe our pipeline of drug candidates represent a significant market opportunity. For instance, according to Visiongain, the world rheumatoid arthritis market size is predicted to generate revenues of $34.6 billion in 2020 and the psoriasis drug market is forecasted to be worth $11.4 billion by 2020. According to DelveInsight, the HCC drug market in the G8 countries (U.S., Germany, France, Italy, Spain, UK, Japan and China) is expected to reach $3.8 billion by 2027.
We have in-licensed an allosteric modulator of the A3AR, CF602 from Leiden University. In addition, we have out-licensed the following:
| ● | Piclidenoson for the treatment of (i) rheumatoid arthritis to Kwang Dong Pharmaceutical Co. Ltd., or KD, for Korea, (ii) psoriasis and rheumatoid arthritis to Cipher Pharmaceuticals for Canada, (iii) rheumatoid arthritis and psoriasis to Gebro Holding, or Gebro, for Spain, Switzerland and Austria, and (iv) rheumatoid arthritis and psoriasis to CMS Medical Venture Investment or CMS Medical, for China (including Hong Kong, Macao and Taiwan); and |
| ● | Namodenoson for the treatment of (i) liver cancer and NASH to Chong Kun Dang Pharmaceuticals, or CKD, for South Korea, and (ii) advanced liver cancer and NAFLD/NASH to CMS Medical for China (including Hong Kong, Macao and Taiwan). |
We believe that our drug candidates have certain unique characteristics and advantages over drugs currently available on the market and under development to treat these indications. To date, we have generated our pipeline by in-licensing, researching and developing two synthetic A3AR agonists, Piclidenoson and Namodenoson, and an allosteric modulator, CF602. For example, our technology platform is based on the finding that the A3AR is highly expressed in pathological cells, such as various tumor cell types and inflammatory cells. High A3AR expression levels are also found in peripheral blood mononuclear cells, or PBMCs, of patients with cancer, inflammatory and viral diseases. PBMCs are a critical part of the immune system required to fight infection. We believe that targeting the A3AR with synthetic and highly selective A3AR agonists, such as Piclidenoson and Namodenoson, and allosteric modulators, such as CF602, induces anti-cancer and anti-inflammatory effects. In addition, our human clinical data suggests that the A3AR is a biological marker and that high A3AR expression prior to treatment may be predictive of good patient response to our drug treatment. In fact, as a result of our research we have developed a simple blood assay to test for A3AR expression as a predictive biological marker. We have been granted a U.S. patent with respect to the intellectual property related to such assay and utilized this assay in our Phase IIb study of Piclidenoson for the treatment of rheumatoid arthritis.
33
Moreover, we believe characteristics of Piclidenoson, as exhibited in our clinical studies to date, including its good safety profile, clinical activity, simple and less frequent delivery through oral administration and its low cost of production, position it well against the competition in the autoimmune-inflammatory markets, including the rheumatoid arthritis and psoriasis markets, where treatments, when available, often include injectable drugs, many of which can be highly toxic, expensive and not always effective. Furthermore, pre-clinical pharmacology studies in different experimental animal models of arthritis revealed that Piclidenoson acts as a disease modifying anti-rheumatic drug, or a DMARD, which, when coupled with its good safety profile, makes it competitive in the psoriasis and rheumatoid arthritis markets. Our recent findings also demonstrate that a biological predictive marker can be utilized prior to treatment with Piclidenoson, which may allow it to be used as a personalized medicine therapeutic approach for the treatment of rheumatoid arthritis.
Like Piclidenoson, Namodenoson has a good safety profile, is orally administered and has a low cost of production, which we believe positions it well in the HCC market, where only one drug, Nexavar, has been approved by the FDA. In addition, pre-clinical studies show Namodenoson’s novel mechanism of action which entails de-regulation of three key signaling pathways which mediate the etiology and pathology of NAFLD/NASH and are responsible for the anti-inflammatory and anti-fibrogenic effect in the liver. Most recently, pre-clinical data support Namodenoson’s potential utilization as an anti-obesity drug.
Nevertheless, other drugs on the market, new drugs under development (including drugs that are in more advanced stages of development in comparison to our drug candidates) and additional drugs that were originally intended for other purposes, but were found effective for purposes targeted by us, may all be competitive to the current drugs in our pipeline. In fact, some of these drugs are well established and accepted among patients and physicians in their respective markets, are orally bioavailable, can be efficiently produced and marketed, and are relatively safe. None of our product candidates have been approved for sale or marketing and, to date, there have been no commercial sales of any of our product candidates.
Our research further suggests that A3AR affects pathological and normal cells differently. While specific A3AR agonists, such as Piclidenoson and Namodenoson, and allosteric modulators, such as CF602, appear to inhibit growth and induce apoptosis of cancer and inflammatory cells, normal cells are refractory, or unresponsive to the effects of these drugs. To date, the A3AR agonists have had a positive safety profile as a result of this differential effect.
We are currently: (i) conducting a Phase III trial for Piclidenoson in the treatment of rheumatoid arthritis, (ii) conducting a Phase III trial for Piclidenoson in the treatment of psoriasis, (iii) completing the analysis of the results of our Phase II advanced liver cancer study having recently released top-line results, (iv) conducting a Phase II trial of Namodenoson in the treatment of NASH with top-line results expected in the second half of 2019, and (v) investigating additional compounds, targeting the A3 adenosine receptor, for the treatment of sexual dysfunction and have therefore postponed a planned Investigational New Drug (IND) submission for this indication.
34
Our Strategy
Our strategy is to build a fully integrated biotechnology company that discovers, in-licenses and develops an innovative and effective small molecule drug portfolio of ligands that bind to a specific therapeutic target for the treatment of cancer, liver and inflammatory disease and sexual dysfunction. We continue to develop and test our existing pipeline, while also testing other indications for our existing drugs and examining, from time to time, the potential of other small molecules that may fit our platform technology of utilizing small molecules to target the A3AR. We generally focus on drugs with global market potential and we seek to create global partnerships to effectively assist us in developing our portfolio and to market our products. Our approach allows us to:
| ● | continue to advance our clinical and preclinical pipeline; |
| ● | test our products for additional indications which fit our molecules’ mechanism of action; |
| ● | identify other small molecule drugs or ligands; |
| ● | focus on our product candidates closest to realizing their potential; and |
| ● | avoid dependency on a small number of small molecules and indications. |
Using this approach, we have successfully advanced our product candidates for a number of indications into various stages of clinical development. Specific elements of our current strategy include the following:
Successful development of our existing portfolio of small molecule orally bioavailable drugs for the treatment of various diseases. We intend to continue to develop our existing portfolio of small molecule orally bioavailable drugs, both for existing targeted diseases, as well as other potential indications. Our drug development will continue to focus on cancer, liver and inflammatory disease and sexual dysfunction. We intend to focus most prominently on advancing our product candidates that are in the most advanced stages, i.e., rheumatoid arthritis and psoriasis with respect to Piclidenoson, and HCC and NASH with respect to Namodenoson.
Use our expertise with our platform technology to evaluate in-licensing opportunities. We continuously seek attractive product candidates and innovative technologies to in-license or acquire. We intend to focus on product candidates that would be synergistic with our A3AR expertise. We believe that by pursuing selective acquisitions of technologies in businesses that complement our own, we will be able to enhance our competitiveness and strengthen our market position. We intend to utilize our expertise in A3AR and our pharmacological expertise to validate new classes of small molecule orally bioavailable drugs. We will then seek to grow our product candidate portfolio by attempting to in-license those various candidates and to develop them for a variety of indications.
Primarily develop products that target major global markets. Our existing product candidates are almost all directed at diseases that have major global markets. Our intent is to continue to develop products that target diseases that affect significant populations using our platform technology. We believe these arrangements will allow us to share the high development cost, minimize the risk of failure and enjoy our partners’ marketing capabilities, while also enabling us to treat a more significant number of persons. We believe further that this strategy will increase the likelihood of advancing clinical development and potential commercialization of our product candidates.
Commercialize our product candidates through out-licensing arrangements. We have entered into several out-licensing arrangements with leading pharmaceutical companies in the Far East, Canada and Europe. We intend to continue to commercialize our product candidates through out-licensing arrangements with third parties who may perform any or all of the following tasks: completing development, securing regulatory approvals, manufacturing, marketing and sales. We do not intend to develop our own manufacturing facilities or sales forces. If appropriate, we may enter into co-development and similar arrangements with respect to any product candidate with third parties or commercialize a product candidate ourselves. We believe these arrangements will allow us to share the high development cost, minimize the risk of failure and enjoy our partners’ marketing capabilities. We believe further that this strategy will increase the likelihood of advancing clinical development and potential commercialization of our product candidates.
35
Our Product Pipeline
The table below sets forth our current pipeline of product candidates, including the target indication and status of each.
| Clinical Application/Drug | Pre-Clinical | Phase I | Phase II | Phase III | |||||
| Autoimmune-Inflammatory | |||||||||
| Rheumatoid Arthritis - Piclidenoson (1) | |||||||||
| Psoriasis - Piclidenoson (2) | |||||||||
| Oncology/Liver diseases | |||||||||
| HCC – Namodenoson (3) | |||||||||
| NASH – Namodenoson (4) | |||||||||
| Sexual Dysfunction - CF602 (5) | |||||||||
| Completed | |||||||||
| On-going | |||||||||
| Preparatory work | |||||||||
| (1) | We are conducting a Phase III trial for Piclidenoson in the treatment of rheumatoid arthritis. |
| (2) | We are conducting a Phase III trial for Piclidenoson in the treatment of psoriasis. |
| (3) | We recently announced top-line results of a Phase II study with respect to the development of Namodenoson for the treatment of HCC with approximately 78 patients. |
| (4) | We are conducting a Phase II trial of Namodenoson in the treatment of NASH. Top-line results are expected in the second half of 2019. |
| (5) | We are investigating additional compounds, targeting the A3AR, for the treatment of sexual dysfunction and have therefore postponed a planned IND submission for this indication. |
36
Piclidenoson (CF101)
Piclidenoson, our lead therapeutic product candidate, is in development for the treatment of autoimmune-inflammatory diseases. In certain of our pharmacological studies, Piclidenoson has also shown potential for development for the treatment of Crohn’s disease. Piclidenoson is a highly-selective, orally bioavailable small molecule synthetic drug, which targets the A3AR. Based on our clinical studies to date, we believe that Piclidenoson has a favorable safety profile and significant anti-inflammatory effects as a result of its capability to inhibit the production of inflammatory cytokines, such as TNF-α, IL-6 and IL-1, and chemokines, or small cytokines, such as MMPs, by signaling key proteins such as NF-кB and PKB/AKT. Overall, these up-stream events result in apoptosis of inflammatory cells. See Figure 1 below. Piclidenoson’s anti-inflammatory effect is mediated via the A3AR, which is highly expressed in inflammatory cells.

Figure 1: Piclidenoson anti-inflammatory mechanism of action
Set forth below are general descriptions of the inflammatory diseases with respect to which Piclidenoson is currently undergoing, or is in preparation for clinical trials.
Rheumatoid Arthritis: Rheumatoid arthritis is a chronic, systemic autoimmune-inflammatory disease that may affect many tissues and organs, but principally attacks flexible synovial, or joints, on both sides of the body. This symmetry helps distinguish rheumatoid arthritis from other types of arthritis, which is the general term for joint inflammation. Although the cause of rheumatoid arthritis is unknown, autoimmunity plays a pivotal role in both its chronicity and progression. The disease involves abnormal B cell–T cell interaction, which results in the release of cytokines. The cytokines signal the release of inflammatory cells. The inflammatory cells migrate from the blood into the joints and joint-lining tissue. There, the cells produce inflammatory substances that cause irritation, wearing down of cartilage, or the cushioning material at the end of bones, swelling and inflammation of the joint lining, which is caused by excess synovial fluid, the development of pannus, or fibrous tissue, in the joint, and ankylosis, or fusion of the joints. Joint inflammation is characterized by redness, warmth, swelling and pain within the joint. As the cartilage wears down, the space between the bones narrows. If the condition worsens, the bones could rub against each other. As the lining expands due to inflammation from excess fluid, it may erode the adjacent bone, resulting in bone damage. Rheumatoid arthritis can also produce diffuse inflammation in the lungs, membrane around the heart, the membranes of the lungs, and white of the eye, and also nodular lesions, most common in subcutaneous tissue.
Psoriasis: Psoriasis is an autoimmune hereditary disease that affects the skin. In psoriasis, immune cells move from the dermis to the epidermis, where they stimulate keratinocytes, or skin cells, to proliferate. DNA acts as an inflammatory stimulus to stimulate receptors which produce cytokines, such as IL-1, IL-6, and TNF-α, and antimicrobial peptides. These cytokines and antimicrobial peptides signal more inflammatory cells to arrive and produce further inflammation. In other words, psoriasis occurs when the immune system overreacts and mistakes the skin cells as a pathogen, and sends out faulty signals that speed up the growth cycle of skin cells. Normally, skin cells grow gradually and flake off approximately every four weeks. New skin cells grow to replace the outer layers of the skin as they shed. But in psoriasis, new skin cells move rapidly to the surface of the skin in days rather than weeks. They build up and form thick patches called plaques.
37
There are five types of psoriasis: plaque, guttate, inverse, pustular and erythrodermic. The most common form, plaque psoriasis, is commonly seen as red and white hues of scaly patches appearing on the top first layer of the epidermis, or skin. In plaque psoriasis, skin rapidly accumulates at these sites, which gives it a silvery-white appearance. Plaques frequently occur on the skin of the lower back, elbows and knees, but can affect any area, including the scalp, palms of hands, soles of feet and genitals. The plaques range in size from small to large. In contrast to eczema, psoriasis is more likely to be found on the outer side of the joint. Some patients, though, have no dermatological symptoms.
Psoriasis is a chronic recurring condition that varies in severity from minor localized patches to complete body coverage. Fingernails and toenails are frequently affected, known as psoriatic nail dystrophy, and can be seen as an isolated symptom. Psoriasis can also cause inflammation of the joints, which is known as psoriatic arthritis.
Pre-Clinical Studies of Piclidenoson
The information below is based on the various studies conducted with Piclidenoson, including preclinical studies. All of the studies were conducted by Can-Fite and/or by Can-Fite’s partners or affiliates.
Pre-clinical studies are a set of experiments carried out in animals to show that a certain drug does not evoke toxicity. Based on the animal studies and safety data, one can approach the FDA and request permission to conduct a Phase I study in human beings.
The toxicity of Piclidenoson has been evaluated following 28-day, 90-day, six-month and nine-month good laboratory practice repeated-dose toxicity studies in male and female mice (28-day, 90-day and six-month), dogs (single-dose only), and monkeys (28-day, 90-day and nine-month). Even though the dose of Piclidenoson in these studies was escalated to an exposure that is many folds higher than the dose used in human clinical studies, no toxic side effects were identified.
Effects on cardiovascular parameters were evaluated in conscious instrumented monkeys and anesthetized dogs. These studies demonstrated no significant cardiovascular risk.
Genotoxicity studies were conducted in bacterial and mammalian mutation assays in vitro (i.e., laboratory) and in an in vivo (i.e., animal) mouse micronucleus assay. These studies were all negative, indicating no deleterious action on cellular genetic material.
Reproductive toxicology studies that we completed in mice and rabbits did not reveal evidence of negative effects on male or female fertility. In mouse teratology studies, or studies for abnormalities of physiological developments, craniofacial and skeletal abnormalities were observed at doses greater than 10 mg/kg; however, no such effects were observed at 3 mg/kg demonstrating the safety of the drug in this concentration range. Teratogenicity, or any developmental anomaly in a fetus, was not observed in rabbits given doses (greater than 13 mg/kg) that induced severe maternal toxicity in such rabbits.
Studies of P450 enzymes, or enzymes that participate in the metabolism of drugs, showed that Piclidenoson caused no P450 enzyme inhibition, or increased drug activity, or induction, or reduced drug activity. Studies carried out with radiolabeled (C14) Piclidenoson in rats showed that the drug is excreted essentially unchanged. These studies also showed that the drug is widely distributed in all body parts, except the central nervous system.
Clinical Studies of Piclidenoson
The information below is based on the various studies conducted with Piclidenoson, including clinical studies in patients with autoimmune-inflammatory and ophthalmic diseases. All of the studies were conducted by Can-Fite and/or by Can-Fite’s partners or affiliates.
Phase I Clinical Studies of Piclidenoson
Piclidenoson has been studied comprehensively in normal volunteer trials to assess safety, pharmacokinetic metabolism and food interaction. Two Phase I studies in 40 healthy volunteers, single dose and repeated dose, indicated that Piclidenoson is rapidly absorbed (reaching a maximal concentration within one to two hours) with a half-life of eight to nine hours. Some mild adverse events (principally, increased heart rate) were observed at doses higher than single doses of 10.0 mg and twice-daily doses of 5.0 mg. Such increase in heart rate was not accompanied by any change in QT intervals. The drug showed linear kinetics, in that the concentration that results from the dose is proportional to the dose and the rate of elimination of the drug is proportional to the concentration, and low inter-subject variability, meaning that the same dose of the drug does not produce large differences in pharmacological responses in different individuals. A fed-fast Phase I study (with and without food) demonstrated that food causes some attenuation in Piclidenoson absorption; accordingly, Piclidenoson is administered to patients on an empty stomach in our trials. An additional Phase I study of the absorption, metabolism, excretion and mass balance of 4.0 mg (C14) Piclidenoson was conducted in six healthy male subjects and demonstrated that Piclidenoson was generally well-tolerated in this group.
38
Based on the findings from Phase I clinical studies, 4.0 mg twice daily, or BID, was selected as the upper limit for initial Phase II clinical trials.
Additionally, in preparation for Phase III studies of Piclidenoson to establish cardiac safety in humans prior to registration for marketing approval, we conducted a cardiodynamic trial that was a placebo-controlled crossover study using precise methodology to determine the effect of Piclidenoson on electrocardiograms of healthy volunteers. The primary objective of the trial was to assess whether Piclidenoson causes a delay in cardiac repolarization, as manifested by prolongation of the QT interval of the electrocardiogram. A drug-induced delay in cardiac repolarization creates an electrophysiological environment that can lead to the development of ventricular cardiac arrhythmias. In this study, Piclidenoson doses were up to 3-fold higher than the highest dose expected to be used in our registration-directed clinical trials. Trial results showed that our highest projected Piclidenoson dose had no clinically significant adverse electrocardiographic effects.
Phase II, Phase II/III and Phase III Clinical Studies of Piclidenoson
Piclidenoson has completed eleven Phase II studies, one Phase II/III study and one Phase III study in different clinical indications including psoriasis, rheumatoid arthritis, glaucoma and dry eye syndrome, or DES, in approximately 1,500 patients. These studies indicate that Piclidenoson has a favorable safety profile at doses up to 4.0 mg BID for up to 32 weeks. In these studies, we did not observe a dose-response relationship between Piclidenoson and adverse events. Moreover, we did not observe any clinically significant changes in vital signs, electrocardiograms, blood chemistry or hematology.
Piclidenoson given as a standalone therapy reached the primary endpoint in Phase II clinical studies in DES; however, a Phase III study of Piclidenoson for DES failed to reach the primary endpoint. We have observed positive data utilizing Piclidenoson as a standalone drug in a Phase IIa clinical study in rheumatoid arthritis. In this study, we also observed a significant direct correlation between A3AR expression prior to treatment and the patients’ responses to Piclidenoson. However, we did not fully attain the primary endpoint in this study as we did not observe a significant difference in responses between Piclidenoson and the placebo (which for this study was 0.1 mg of Piclidenoson). Moreover, two Phase IIb studies in rheumatoid arthritis utilizing Piclidenoson in combination with MTX, also failed to reach the primary endpoints. Based on this data, we believe that the failures in the Phase IIb studies in rheumatoid arthritis may have been due to low A3AR expression in the MTX-treated patients. A Phase IIb of Piclidenoson given as a standalone therapy in patients with A3AR expression levels above a certain threshold reached the primary endpoint in rheumatoid arthritis in December 2013. Piclidenoson has been tested in Phase II trials to establish dose and activity (first, orally administered capsules and then tablets in formulations of 1.0, 2.0 and 4.0 mg of Piclidenoson BID) in psoriasis (moderate to severe plaque psoriasis), rheumatoid arthritis and DES (moderate to severe). A Phase II/III study of Piclidenoson for psoriasis did not meet its primary endpoint although positive data from further analysis of the Phase II/III study suggests Piclidenoson as a potential systemic therapy for patients with moderate-severe psoriasis. In addition, a Phase II study of Piclidenoson for glaucoma showed no statistically significant differences between the Piclidenoson treated group and the placebo group in the primary endpoint of lowering IOP.
Psoriasis: The rationale for utilizing Piclidenoson to treat psoriasis stems from our pre-clinical pharmacology studies showing that Piclidenoson acts as an anti-inflammatory agent via the inhibition of inflammatory cytokines, including TNF-α, which plays a major role in the pathogenesis of psoriasis. In addition, the A3AR is over-expressed in the tissue and PBMCs of patients with psoriasis.
39
We completed an exploratory Phase II trial in ten European and Israeli medical centers involving 76 patients. This study was a randomized, double-blind, placebo controlled and included four cohorts of 1.0, 2.0, and 4.0 mg of Piclidenoson and a placebo for a 12-week period. The study objectives were efficacy and safety of daily doses of Piclidenoson administered orally in patients with moderate-to-severe plaque-type psoriasis and the efficacy endpoints were improvements in both the Psoriasis Area Sensitivity Index score, or PASI score, and the Physicians’ Global Assessment score, or PGA score. We concluded that Piclidenoson met such efficacy endpoints and was well tolerated and effective in ameliorating disease manifestations in these patients. The patient group receiving 2.0 mg Piclidenoson BID showed progressive improvement over the course of the 12-week study in the PGA and PASI scores. Analysis of the mean change from baseline in the PASI score at week 12 revealed a statistically significant difference between the 2.0 mg Piclidenoson BID treated group and the placebo group (p<0.001 versus baseline and p=0.031 versus placebo). Analysis of the PGA score revealed that 23.5% of the patients treated with the 2.0 mg Piclidenoson BID achieved a score of 0 or 1, in comparison to 0% in the placebo group (p<0.05). The study also demonstrated linear improvement in patients in both PASI and PGA. See Figure 2. No drug-related serious adverse events were evident during the study.
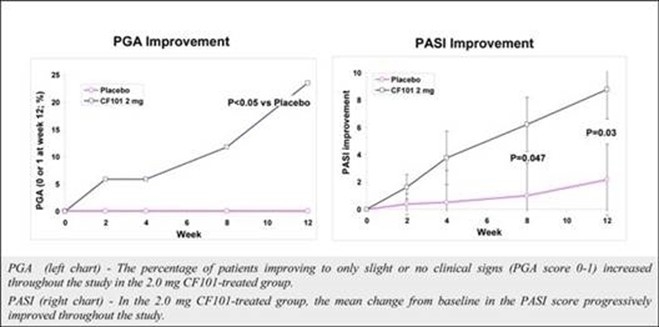
Figure 2: Psoriasis efficacy by PGA and PASI
40
Set forth below are representative pictures of a patient with plaque-type psoriasis on the upper and lower back treated with 2.0 mg Piclidenoson BID, both baseline and week 12.
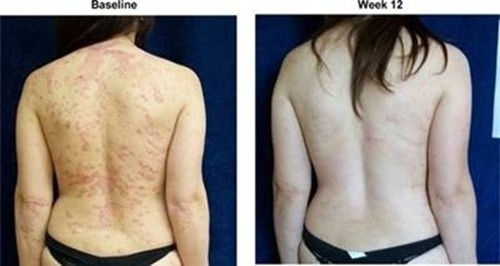
A comparison between baseline and week 12 of a patient treated with 2.0 mg CF101
In February 2015, we completed a Phase II/III randomized, double-blind, placebo-controlled, dose-finding study of the efficacy and safety of Piclidenoson administered daily orally in patients with moderate-to-severe plaque psoriasis. This clinical trial enrolled 326 patients in 17 clinical centers in the United States, Europe and Israel, of which 103 patients were enrolled in the first study cohort and were treated for 6 months and 223 patients were enrolled in the second study cohort and were treated for 8 months. The first study cohort was comprised of three arms with patients receiving: 1.0 mg of Piclidenoson; 2.0 mg of Piclidenoson; and placebo. All patients receiving placebo were switched to either 1.0 mg or 2.0 mg of Piclidenoson after 12 weeks. Based on a positive safety and efficacy interim analysis of the first 103 patients who completed 24 weeks of treatment in the trial, we decided to continue patient enrollment for the second stage of the study and the study protocol was amended to extend the Piclidenoson 2.0 mg BID and placebo administration for a period of 32 weeks. The positive clinical effects of the Piclidenoson 2.0 mg BID dose relative to a placebo were observed in a variety of standard psoriasis assessment parameters, including PASI 75 and PGA scores, with the responses accumulating steadily over the 24-week treatment period.
In March 2015, we announced the study did not meet its primary endpoint of a statistically significant improvement in the PASI 75 score relative to placebo after 12 weeks of treatment. Further analysis of the entire study period revealed that by 32 weeks of treatment with Piclidenoson, 33% of the patients achieved PASI 75 while the mean percent of improvement in PASI score was 57% (p<0.001). This was a statistically significant cumulative and linear improvement during weeks 16 to 32. Most significantly, by week 32 of the study, 20% of the study patients reached PASI 90, a result demonstrating a response rate of 90% clearing of skin lesions. PASI 90 is one of the most stringent and difficult to meet clinical endpoints for measuring responses to psoriasis treatments. Moreover, the PASI 90 subset analysis further suggests a higher and significant (p=0.026) Piclidenoson response rate of 27% among patients previously untreated with systemic psoriasis therapy compared to patients pre-treated with systemic drugs. We believe this presents the opportunity that Piclidenoson can be developed as a first-line systemic therapy for patients with moderate-severe psoriasis and for patients who do not want to be treated with the current systemic drugs due to safety issues.
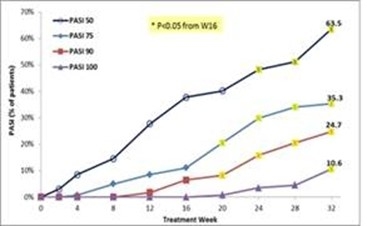
Figure 3: Linear Effect of Piclidenoson on PASI Scores through 32 Weeks of Treatment
41
We are currently conducting our pivotal COMFORT Phase III trial for Piclidenoson for the treatment of psoriasis. The trial is a randomized, double-blind, placebo- and active-controlled study that is investigating the efficacy and safety of daily Piclidenoson 2.0 mg or 3.0 mg administered twice daily orally as compared to placebo as its primary endpoint and as compared to apremilast (Otezla®) as a secondary endpoint in approximately 400 patients with moderate-to-severe plaque psoriasis. Medication is to be taken orally twice daily for 32 weeks in a double-blinded fashion. The primary end point is the proportion of subjects who achieve a PASI score response of ≥75% (PASI 75) vs. placebo at week 16. The secondary endpoints include non-inferiority to Otezla® on weeks 16 and 32, achievement of PASI 50 at week 16 and efficacy and safety data for Piclidenoson through the extension period of up to 48 weeks of treatment. Patients are being selected to the study based on over expression of the A3AR biomarker. In August 2018, we announced enrollment of the first patient. We expect COMFORT will serve as the first of two pivotal studies required for EMA-drug approval.
Rheumatoid Arthritis: We conducted a Phase IIa blinded to dose study in 74 patients with rheumatoid arthritis, randomized to receive Piclidenoson as a monotherapy in one of three doses—0.1 mg, 1.0 mg and 4.0 mg. The primary efficacy endpoint was ACR20 response at week 12, a criterion determined by the American College of Rheumatology that reflects 20% improvement in inflammation parameters. The study data revealed maximal response at the 1.0 mg group, showing 55.6% with ACR20, 33.3% with 50% improvement, or ACR50, and 11.5% with 70% improvement, or ACR70. Piclidenoson administered BID for 12 weeks resulted in improvement in signs and symptoms of rheumatoid arthritis and was well-tolerated. See Figure 4. Studies in the United States were conducted pursuant to an open IND, which was received by the FDA in 2005.
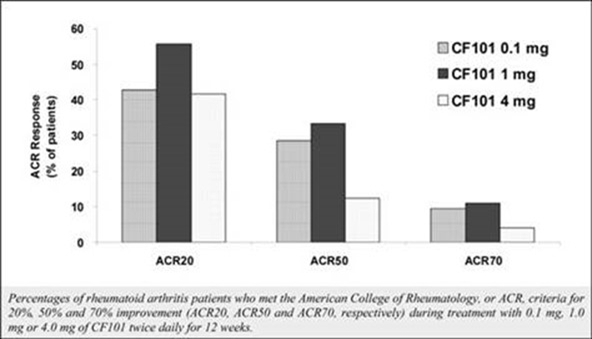
Figure 4: Rheumatoid Arthritis efficacy by ACR
Subsequently, two Phase IIb studies with Piclidenoson in combination with MTX were conducted. The study protocols were multicenter, randomized, double-blind, placebo-controlled, parallel-group and dose-finding to determine the safety and efficacy of daily Piclidenoson administered orally when added to weekly MTX in patients with active rheumatoid arthritis. The objectives of both studies were improvement in ACR20, ACR50, ACR70 and DAS28, or the Disease Activity Score of 28 Joints, and EULAR, or the European League Against Rheumatism, response criteria, as well as a positive safety profile. The trials’ primary endpoints were both ACR20.
The first Phase IIb trial showed that the combined treatment had an excellent safety profile, but no significant ACR20 response was observed between the rheumatoid arthritis group treated with Piclidenoson and MTX and the group treated with MTX alone (the placebo group). However, the ACR50, ACR70 and the EULAR Good Values in the combined treatment group were higher than those of the MTX placebo group. The study also indicated that the 1.0 mg Piclidenoson dose was the most favorable dose, i.e., the dose yielded the highest ACR50 and EULAR Good Values as compared to the MTX placebo group. The most commonly reported adverse events in this study included nausea, dizziness, headache and common bacterial and viral infections and infestations.
Following a decision of our Clinical Advisory Board in October 2007, an additional Phase IIb study was initiated. This study was conducted in medical centers in Europe and Israel and included 230 patients who received the drug orally BID (0.1 and 1.0 mg Piclidenoson tablets plus MTX versus a placebo, which was MTX alone) for 12 weeks. On April 30, 2009, we published preliminary results of the Phase IIb study, which were later confirmed as the final results, also indicating that the study’s objectives were not achieved. The most commonly reported adverse events in this study included nausea, myalgia and dizziness.
42
The two Phase IIb studies failed to achieve the primary endpoint of ACR20. A cross study analysis of the three rheumatoid arthritis clinical studies revealed that in the first Phase IIa study, where Piclidenoson had been administered as a standalone drug, A3AR had been over-expressed in the patients’ PBMCs prior to Piclidenoson treatment, whereas A3AR had not been over-expressed in the Phase IIb patient population. We believe, based on the foregoing data, that there may be a direct and statistically significant correlation between A3AR over-expression at baseline and patients’ response to Piclidenoson, and that Piclidenoson should be administered as a standalone drug and not in combination with MTX. Furthermore, the correlation between A3AR expression levels prior to treatment and patients’ response to the drug suggest that the A3AR may be a predictive biomarker to be analyzed prior to Piclidenoson treatment. See Figures 5 and 6.
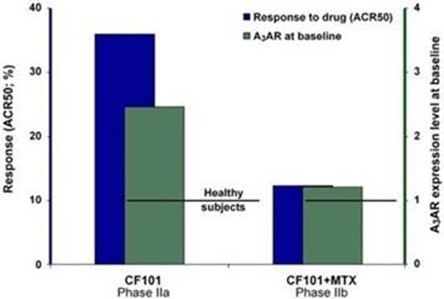
Figure 5: Direct correlation between A3AR at baseline and response to Piclidenoson
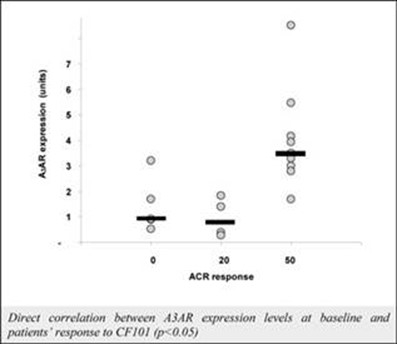
Figure 6: Direct correlation between A3AR at baseline and response to Piclidenoson
43
Based on the results of the two Phase IIb studies, we conducted an additional Phase IIb clinical study with Piclidenoson as a stand-alone, monotherapy treatment and not in combination with MTX. The trial was a 12-week multicenter, randomized, double-blind, placebo-controlled, parallel-group study involving 79 patients to determine the safety and efficacy of Piclidenoson administered orally daily in patients with active rheumatoid arthritis and elevated baseline expression levels of the A3AR in PBMCs. Enrolled patients had high baseline A3AR biomarker expression (determined at 1.5-fold over a predetermined age-matched standard). This selection criteria was made following the findings during previous Phase IIa and IIb rheumatoid arthritis studies showing a positive correlation between A3AR expression at baseline and patients’ response to the drug, potentially rendering A3AR expression as a predictive biomarker. The primary objectives of this study were to determine the efficacy of oral Piclidenoson when administered daily as a standalone treatment for 12 weeks to patients with active rheumatoid arthritis and elevated baseline expression levels of the A3AR in the patients’ PBMCs, in comparison to a placebo treatment, and to assess the safety of daily oral Piclidenoson under the circumstances of the trial. In December 2013, we announced the results of the study in which Piclidenoson met all primary efficacy endpoints, showing statistically significant superiority over placebo in reducing signs and symptoms of rheumatoid arthritis as compared to the placebo. The treatment had an ACR20 response rate of 49% for Piclidenoson compared to 25% for placebo (p=0.035), an ACR50 response rate of 19% for Piclidenoson compared to 9% for placebo, and an ACR70 response rate of 11% for Piclidenoson compared to 3% for placebo. See Figure 7. Similar to our observations in the previously reported Piclidenoson psoriasis trials, the response of patients with rheumatoid arthritis was cumulative over time, suggesting a consistent anti-inflammatory effect of Piclidenoson. Moreover, half of the rheumatoid arthritis patients treated with Piclidenoson showed clinically meaningful improvement. Piclidenoson was very well-tolerated and showed no evidence of immunosuppression, and there were no severe treatment-emergent adverse events during the study. A subgroup analysis of 16 patients with no prior systemic therapy showed a dramatic increase in the response showing ACR20 of 75%, ACR50 of 50%, and ACR70 of 50%. See Figure 7. We believe this may be related to the fact that in this patient population there is a full receptor expression since they had not been treated earlier with any systemic drugs.
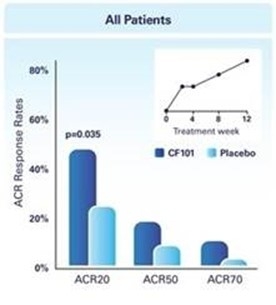 |
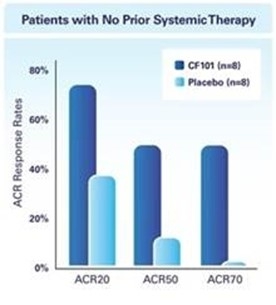 |
Figure 7: ACR response data – Rheumatoid Arthritis phase IIb
We are currently conducting our pivotal ACRobat Phase III trial of Piclidenoson to evaluate Piclidenoson as a first line treatment and replacement for MTX. The trial is a randomized, double-blind, active and placebo-controlled, parallel-group study in approximately 500 patients in Europe, Israel and Canada. The primary endpoint of ACRobat is low disease activity after 12 weeks of treatment in patients dosed with Piclidenoson compared to those dosed with MTX. Piclidenoson at 1.0 mg and 2.0 mg, or placebo, will be administered twice daily, and MTX or placebo will be administered once weekly. Secondary endpoints include disease activity remission at week 24, ACR 20/50/70 response rates, European League Against Rheumatism good and moderate response rates and change from baseline for disease activity and ACR responses. The total study duration will be 24 weeks in order to provide more data on long term efficacy and safety. In the fourth quarter of 2017, we announced the enrollment and dosing of the first patient in the trial. We expect ACRobat will serve as the first of two pivotal studies required for EMA-drug approval.
44
DES: DES is an eye disease caused by eye dryness, which, in turn, is caused by either decreased tear production or increased tear film evaporation. A Phase II study in DES was conducted by Can-Fite after discovering that patients in the Phase IIa study for another condition also experienced improvement in DES symptoms. The results of the Phase II trial demonstrated the ability of Piclidenoson to improve signs of ocular surface inflammation of the patients studied. Following positive results in the Phase II study, we initiated a Phase III DES trial, under an IND with the FDA, which was conducted by OphthaliX in the United States, Europe and Israel. The randomized, double-masked Phase III clinical trial enrolled 237 patients with moderate-to-severe DES who were randomized to receive two oral doses of Piclidenoson (0.1 and 1.0 mg) and a placebo, for a period of 24 weeks. The primary efficacy endpoint was complete clearing of corneal staining. In December 2013, we announced the results of this Phase III study of Piclidenoson for the treatment of DES. In the study, Piclidenoson did not meet the primary efficacy endpoint of complete clearing of corneal staining, nor the secondary efficacy endpoints. Nonetheless, Piclidenoson was found to be well tolerated. In 2014, we decided to end the development of Piclidenoson for the DES indication. This decision was based on a lack of correlation between patients’ response to Piclidenoson and over-expression of the drug target, the A3AR in this patient population.
Glaucoma: Glaucoma is an eye disease in which the optic nerve is damaged. This optic nerve damage involves loss of retinal ganglion cells, or neurons located near the inner surface of the retina, in a characteristic pattern. There are many different subtypes of glaucoma, but they can all be considered to be a type of optic neuropathy. Raised IOP is the most important and only modifiable risk factor for glaucoma. However, some individuals may have high IOP for years and never develop optic nerve damage. This is known as ocular hypertension. Others may develop optic nerve damage at a relatively low IOP, and, thus, glaucoma. Untreated glaucoma can lead to permanent damage of the optic nerve and resultant visual field loss, which over time can progress to blindness. A Phase II clinical trial of Piclidenoson for the treatment of glaucoma was conducted by OphthaliX. The randomized, double-masked, placebo-controlled, parallel-group Phase II clinical trial was designed to evaluate the safety and efficacy of Piclidenoson when administered orally twice daily for up to 16 weeks in patients with elevated IOP. A total of 89 patients were enrolled in the study. The study was conducted with two cohorts. In the first cohort, treatment was randomized in a 3:1 ratio of 1.0 mg Piclidenoson to placebo. In the second cohort, which was also randomized in a 3:1 Piclidenoson to placebo ratio, the Piclidenoson dose was increased to 2.0 mg. In July 2016, top line results were announced. In this trial, no statistically significant differences were found between the Piclidenoson treated group and the placebo group in the primary endpoint of lowering IOP. Piclidenoson was found to have a favorable safety profile and was well tolerated. Based on these overall results, OphthaliX saw no immediate path forward in glaucoma and we have since terminated the License Agreement that we granted to OphthaliX, following the Merger with Wize Pharma. See “Item 4. Information on the Company—A. History and Development of the Company.”
Additional Developments with Piclidenoson
Osteoarthritis
According to the Arthritis Foundation, osteoarthritis, or OA, is the most common arthritic disease. Currently, there is a shortage of effective drugs for treating OA patients. Piclidenoson has induced a significant anti-inflammatory effect in experimental animal models with respect to the treatment of OA and, as such, we are currently preparing for a Phase II study. We have not yet filed an IND for this indication as Piclidenoson for the treatment of OA is not currently being clinically tested in the United States and there are no near-term plans to do so.
Crohn’s Disease
Crohn’s disease is an inflammatory bowel disease that may affect any portion of the gastrointestinal tract, causing a wide variety of symptoms. It primarily causes abdominal pain, diarrhea, vomiting and weight loss; however, it may also cause complications outside the gastrointestinal tract, such as skin rashes, arthritis, inflammation of the eye, tiredness and lack of concentration. Pre-clinical pharmacology studies that we have conducted demonstrated the efficacy of Piclidenoson for the treatment of Crohn’s disease. We do not presently have plans for the treatment of Crohn’s disease.
45
Namodenoson (CF102)
Namodenoson is our second drug candidate and is under development for the treatment of HCC, hepatitis C virus, or HCV, or NAFLD, the precursor to NASH. Namodenoson is also a small, orally bioavailable molecule, and an A3AR agonist, with high affinity and selectivity to the A3AR. In comparison to the expression in adjacent normal liver tissue, the A3AR is over-expressed in tumor tissues of patients with HCC, and the over-expression is also reflected in the patients’ PBMCs. A3AR over-expression in the patients’ tumor cells and PBMCs is attributed to high expression of certain A3AR transcription factors. The binding of Namodenoson to the A3AR results in down-regulation, or a decrease in the quantity of a cellular component, such as the number of receptors on a cell’s surface, of certain A3AR transcription factors. Our studies have shown that this down-regulation leads to apoptosis of HCC cells. In our pre-clinical and clinical studies, Namodenoson demonstrated anti-cancer, anti-viral and liver protective effects. As a result, we believe that Namodenoson can be used to treat a variety of oncological and liver-related diseases and viruses.
In February 2012, the FDA granted an orphan drug status for the active moiety, or the part of the drug that is responsible for the physiological or pharmacological action of the drug substance, of Namodenoson for the treatment of HCC. Subsequently, in October 2015, the EMA granted Namodenoson orphan drug designation for the treatment of HCC.
An orphan drug designation is a special designation for drug approval and marketing. The special designation is granted to companies that develop a given drug for unique populations and for incurable and relatively rare diseases. The FDA orphan drug designation program provides orphan status to drugs and biologics, which are intended for the safe and effective treatment, diagnosis or prevention of rare diseases or disorders that affect fewer than 200,000 people in the United States and in the EU not more than 5 per 10,000. Orphan drug designations have enabled companies to achieve medical breakthroughs that may not have otherwise been achieved due to the economics of drug research and development as this status lessens some of the regulatory burdens, for approval, including statistical requirements for efficacy, safety and stability, in an effort to maintain development momentum. Orphan drug designation also results in additional marketing exclusivity and could result in certain financial incentives.
In September 2015, the FDA granted Fast Track designation to Namodenoson as a second line treatment to improve survival for patients with advanced HCC who have previously received Nexavar (sorafenib). Fast Track, aimed at getting important new drugs that meet an unmet need to patients earlier, is expected to expedite the development of Namodenoson. Drugs that receive Fast Track designation benefit from more frequent meetings and communications with the FDA to review the drug’s development plan to support approval. It also allows us to submit parts of the NDA on a rolling basis for review as data becomes available.
Israel’s Ministry of Health has previously approved Namodenoson for Compassionate Use for HCC.
Set forth below are general descriptions of the diseases with respect to which Namodenoson has underwent or is currently undergoing or being prepared for clinical trials.
HCC: HCC is an oncological disease characterized by malignant tumors that grow on the surface or inside of the liver. This type of tumor is refractory to chemotherapy and to other anti-cancer agents. HCC, like any other cancer, develops when there is a mutation to the cellular machinery that causes the cell to replicate at a higher rate and/or results in the cell avoiding apoptosis. Chronic infections of Hepatitis B and/or C can aid the development of HCC by repeatedly causing the body’s own immune system to attack the liver cells, some of which are infected by the virus. While this constant cycle of damage followed by repair can lead to mistakes during repair which in turn lead to carcinogenesis, this hypothesis is more applicable, at present, to HCV. Chronic HCV causes HCC through cirrhosis. In chronic Hepatitis B, however, the integration of the virus into infected cells can directly induce a non-cirrhotic liver to develop HCC. Alternatively, repeated consumption of large amounts of ethanol can have a similar effect.
Hepatitis C: HCV is an infectious disease affecting primarily the liver, caused by the Hepatitis C virus. The infection is often asymptomatic, but chronic infection can lead to scarring of the liver and ultimately to cirrhosis, which is generally apparent after many years, and chronic liver disease. The virus also increases the chance for HCC development. In some cases, those with cirrhosis will develop liver failure, liver cancer or life-threatening esophageal and gastric varices, or dilated submucosal veins, which can be life-threatening. HCV is spread primarily by blood-to-blood contact often associated with intravenous drug use, poorly sterilized medical equipment, transfusions, and sexual intercourse.
46
NAFLD/NASH: NASH, also called “fatty liver,” is a condition in which fat builds up inside the liver causing inflammation. Prior to the presence of inflammation, the disease is simply referred to as NAFLD, the most common form of liver disorder in the United States. The accumulation of macroglobular fat inside the liver causes oxidative stress that reduces the efficiency of the liver and can lead to increased liver enzymes such as alanine aminotransferase and aspartate aminotransferase. Loss of liver efficiency and oxidative stress leads to inflammation, liver cell ballooning, and the development of NASH. Prolonged inflammation results in cirrhosis (scar tissue), liver failure, or liver cancer. There are currently no drugs approved for the treatment of NASH.
Pre-Clinical Studies with Namodenoson
We conducted several pre-clinical studies demonstrating robust anti-inflammatory, anti-fibrogenic and anti-steatotic effects, supporting the development of Namodenoson for the NAFLD/NASH indication. Furthermore, the results indicated that Namodenoson was very well tolerated.
In pre-clinical studies, we evaluated the toxicity, stability, metabolism and other safety parameters of Namodenoson at doses much higher than the doses that we currently administer to humans in our clinical trials of Namodenoson.
In pre-clinical pharmacology studies, Namodenoson inhibited the growth of HCC via the induction of tumor cell apoptosis. In addition, in collaboration with leading virology labs, we observed that Namodenoson inhibited viral replication of HCV through the down-regulation of viral proteins. Both of these findings served as a basis to further explore development of this drug for HCC.
In a preclinical study, Namodenoson also revealed its capability to improve liver pathology in a NAFLD/NASH animal model. The data showed:
| ● | Namodenoson had a statistically significant reduction in NAFLD activity score compared to vehicle treated group; |
| ● | Namodenoson reduced liver-to-body weight compared to vehicle treated group; |
| ● | Representative photomicrographs of H&E-stained liver sections showed improved pathology in animals receiving Namodenoson vs. vehicle; |
| ● | Namodenoson decreased plasma serum alanine aminotransferase, or ALT, and triglycerides levels compared to vehicle treated group; and |
| ● | Liver sections from the vehicle treated group exhibited severe micro- and macrovesicular fat deposits, ballooning and inflammatory cell infiltration, whereas the Namodenoson treated group showed a significant decrease in steatosis, ballooning and lobular inflammation compared to the vehicle group. |
In further pre-clinical studies conducted, the following was observed:
| ● | In vivo studies showed that Namodenoson protected the liver against ischemic reperfusion manifested by a statistically significant (p<0.05) reduction in key liver enzymes, SGOT and SGPT. In addition, in studies where partial liver hepatectomy was conducted, a 45% increase in the regeneration rate of the remaining liver was observed after Namodenoson treatment, compared to placebo which regenerated only by 24%; |
| ● | In an in vitro study with hepato-stellate cells, Namodenoson inhibited, in a dose dependent manner, the growth and proliferation of the liver cells, supporting an anti-fibrogenic effect of the drug; |
47
| ● | In a CCL4 mouse model of liver fibrosis, Namodenoson induced an anti-inflammatory effect, lower serum levels of ALT, no accumulation of peritoneal fluid (ascites) and reduced fibrosis in liver sections stained by Sirius Red. In addition, liver protein and mRNA extracts revealed a significant decrease of α-SMA (α-smooth muscle actin) demonstrating an anti-fibrotic effect. Furthermore, the expression level of PI3K and p-STAT-1 were markedly decreased as well as the NKT cells; |
| ● | Namodenoson’s anti-inflammatory and anti-fibrogenic effect was also demonstrated in a STAM-NASH mouse model manifested by a marked reduction in NAFLD activity score (NAS) and fibrosis area. Namodenoson treatment induced a decrease in CK-18 levels suggesting hepato-protective effect and at the same time up-regulated adiponectin levels, reflecting anti-fibrogenic and anti-inflammatory effects; | |
| ● | Namodenoson’s novel mechanism of action which entails de-regulation of three key signaling pathways which mediate the etiology and pathology of NAFLD/NASH and are responsible for the anti-inflammatory and anti-fibrogenic effect in the liver. Pre-clinical studies were conducted in hepato-stellate cells in vitro and in an experimental NASH CCL4 model, showing that in both systems, the molecular mechanism of action of Namodenoson was conferred by decreased expression levels of the signaling protein phosphoinositol-3-phosphate, or PI3K, which controls 3 downstream signal transduction pathways, the Wnt, NF-kB and α-SMA, all of which are responsible for liver inflammation and liver fibrosis; | |
| ● | In an experimental non-alcoholic steatohepatitis (NASH) CCL4 model, Namodenoson had a highly significant effect against inflammation, necrosis, fibrosis and biliary hyperplasia, upon oral treatment with the drug. More specifically, the liver enzymes ALT and AST were dramatically reduced and reversed to normal values upon treatment of the NASH bearing animals with Namodenoson; and | |
| ● | Namodenoson showed a significant decrease in lipid production and fat accumulation utilizing 3T3-L1 adipocytes, functioning as lipid producing cells and are also responsible for fat storage. Namodenoson was also shown to inhibit the proliferation of adipocytes, further hampering the expansion of fat producing cells. |
48
Clinical Studies of Namodenoson
The information discussed below is based on the various studies conducted by Can-Fite with Namodenoson, including clinical studies in patients with oncological and liver-related diseases and viruses.
Phase I Clinical Study
Namodenoson completed a Phase I double-blind, randomized, placebo-controlled, ascending single dose trial to evaluate the safety, tolerability, and pharmacokinetics of orally administered Namodenoson in healthy volunteers. The study was conducted in the United States under an open IND. Namodenoson was found to be safe and well-tolerated with a half-life time of 12 hours. See Figure 8.
Figure 8: Half-life of orally administered Namodenoson – Phase I Clinical Study
Phase I/II and Phase II Clinical Studies
HCC/HCV
Namodenoson completed two Phase I/II studies in Israel, one in patients with HCC and another in patients with HCV. The HCC Phase I/II study was an open-label, dose-escalation study evaluating the safety, tolerability, pharmacokinetics and pharmacodynamics of orally administered Namodenoson in patients with advanced HCC. The primary objectives of the study were to determine the safety and tolerability, dose-limiting toxicities, maximum tolerated dose, and recommended Phase II dose of orally administered Namodenoson in patients with advanced HCC; and to assess the repeat-dose pharmacokinetics behavior of Namodenoson in those patients. The secondary objectives were to document any observed therapeutic effect of Namodenoson in patients with HCC and to evaluate the relationship between PBMCs and the A3AR expression at baseline, as a biomarker, and the effects of Namodenoson in patients with HCC. The study included 18 patients, nine of which were also carriers of HCV. The initial dose of Namodenoson was 1.0 mg BID, with planned dose escalations in subsequent cohorts to 5.0 and 25.0 mg BID. This Phase I/II study achieved its objectives, showing a good safety profile, or no material differences versus a placebo with respect to observed and patient-indicated side effects, for Namodenoson and a linear pharmacokinetic drug profile, with no dose-limiting toxicities at any dose level. The median overall survival time for the patients in this study was 7.8 months, which is encouraging data considering that (i) 67% of the patient population in the study had previously progressed on Nexavar, produced by Onyx Pharmaceuticals and Bayer, and that Namodenoson was a second line therapy for these patients and (ii) 28% of the patient population were Child-Pugh Class B patients (patients classified on the Child Pugh scoring system for chronic liver disease as having significantly impaired liver function) whose overall survival time is usually 3.5 to 5.5 months. Accordingly, we may also consider Namodenoson as a drug to be developed for this patient sub-population of Child-Pugh Class B patients. Namodenoson had no adverse effect on routine measures of liver function over a six-month period in 12 patients treated for at least that duration. These findings are consistent with our pre-clinical Namodenoson data which demonstrated a protective effect on normal liver tissue in an experimental model of liver inflammation. As such, Namodenoson may potentially be a safer alternative to patients with cirrhosis and/or hepatic impairment. The study also demonstrated a direct relationship between A3AR expression at baseline and patients’ response to Namodenoson, suggesting A3AR as a predictive biological marker. We also observed a decrease in the viral load of seven out of nine patients who were also carriers of HCV. The most commonly reported adverse events included loss of appetite, ascites, nausea, diarrhea, constipation and pain. However, many of these events are expected in a population of patients with advanced HCC. The most frequently reported drug-related adverse events included diarrhea, fatigue, loss of appetite, pain and weakness.
49
Our second Phase I/II study was a randomized, double-blind, placebo-controlled, dose-escalation study evaluating the safety, tolerability, biological activity, and pharmacokinetics of orally administered Namodenoson in 32 subjects with chronic HCV genotype 1. Eligible subjects were assigned in a 3:1 ratio (eight subjects in each cohort) to receive QD or BID treatment (1.0, 5.0 and 25.0 mg of Namodenoson) for 15 days with oral Namodenoson or with a placebo. Dose escalation occurred in four sequential cohorts. The study’s primary objectives were to determine the safety and tolerability of orally administered Namodenoson in patients with chronic HCV genotype 1, to assess the effects on HCV load during 15 days of treatment with Namodenoson and to assess the repeat-dose pharmacokinetic behavior of Namodenoson under the conditions of this trial. The secondary objective of this trial was to perform an exploratory evaluation of the relationship between A3AR in PBMCs at baseline and the clinical effects of Namodenoson on the study’s patients. Following the decrease in HCV load that had been observed in HCV patients treated with Namodenoson in the parallel HCC study and the good safety profile of Namodenoson, we received Israeli Institutional Review Board, or IRB, approval to extend the treatment period of the Phase I/II in patients with HCV to four months with the 1.0 mg dose vs. the placebo. The results of this Phase I/II HCV study demonstrated a good safety profile and a linear pharmacokinetic drug profile, however, no significant decrease in the viral load was observed. Notwithstanding, we did observe in the parallel HCC study that seven out of the nine patients with both HCC and HCV experienced a decrease in viral load and that these seven patients were treated with higher Namodenoson dosages than what was administered to the patients with chronic HCV genotype 1 only, and not HCC, possibly explaining the difference in results. The most commonly reported adverse events included loss of appetite, ascites, nausea, diarrhea, constipation and pain. However, many of these events are expected in a population of patients with advanced HCV. The most frequently reported drug-related adverse events included diarrhea, fatigue, loss of appetite, pain and weakness.
We are conducting a Phase II study in HCC patients. In January 2013, as part of our preparatory work for such study, we announced that we believe that the optimal drug dose for the upcoming study is Namodenoson 25.0 mg. This dose was found to be the most effective dose out of the three dosages tested (1.0 mg, 5.0 mg and 25.0 mg) in the previous Phase I/II study. We filed a patent application protecting such optimal dose of Namodenoson for HCC. A publication summarizing the results of the Phase I/II study was published in “The Oncologist,” a leading oncology scientific journal. We also highlighted that one patient has been treated with Namodenoson for over five years. Also as part of the Phase II study, we plan to examine the viral load of HCC patients who are also infected with HCV. If we observe a decrease in the viral load in the HCV sub-population during this forthcoming study, we intend to commence a separate Phase II study for the HCV indication.
The Phase II study is a randomized, double-blind, placebo controlled trial conducted in the United States, Europe and Israel to evaluate the efficacy and safety of Namodenoson as a second-line treatment for advanced HCC in subjects with Child-Pugh B who failed Nexavar as a first line treatment. Advanced HCC in patients with underlying cirrhosis is categorized into three subclasses based on the severity of cirrhosis, starting with Child Pugh A, or CPA, mostly treated with Nexavar and progressing to Child Pugh B, or CPB, and Child Pugh C, or CPC, for which there are no drugs on market with proven efficacy. In the study, we enrolled only patients with CPB stage liver cancer with CBP stage patients being further divided into three categories of increasing severity, namely CPB7, CPB8, and CPB9. These patients already failed first line Nexavar and were treated with Namodenoson (25mg), or placebo, as a second line treatment, twice daily, using a 2:1 randomization. The primary endpoint of the study was median overall survival. Secondary endpoints included progression free survival, partial response, and disease control rate. In March 2014, the study protocol was approved by the IRB at the Rabin Medical Center in Israel and in December 2014, we dosed the first patient at the study’s Israeli site. In the third quarter of 2017, we announced that we completed enrollment and randomization of all 78 patients and in March 2019, we announced top-line results.
While the study did not achieve the primary end point of overall survival in the whole population (n=78), superiority in overall survival was found in the largest study subpopulation of CPB7 (n=56) and in secondary end points in the whole population, including objective response measured by CT or MRI. Findings from the study include the following: (i) for the whole population (n=78), median overall survival was 4.1 months for Namodenoson vs. 4.3 months for placebo (HR: 0.82), (ii) pre-planned subpopulation analysis of the CPB7 patients (n=56), revealed that the Namodenoson treated group (n=34) showed median overall survival of 6.8 months vs 4.3 months in placebo (n=22) [HR: 0.77 (95% CI 0.49-1.40)]. Similarly, for this subgroup of patients, progression free survival was 3.5 months for the Namodenoson treated group vs 1.9 (HR: 0.87) in the placebo group; (iii) objective response in the whole patient population measured by CT or MRI, demonstrated that 9% treated by Namodenoson achieved partial response vs 0% in the placebo group, (iv) consistent with safety results from previously completed clinical trials, Namodenoson was generally well-tolerated, with no treated patients being withdrawn for toxicity and no cases of treatment-related deaths, (v) disease control rate was 18.0% in the Namodenoson group vs 7.1% in the placebo group (p=0.013) after four months of treatment, (vi) 32.0% of patients treated with Namodenoson completed at least 12 months of treatment vs 14.3% who were treated with placebo (p=0.058), (vii) as of March 26, 2019, two patients in the Namodenoson group are ongoing after 19 and 28 months of treatment, respectively. These patients will continue to receive Namodenoson, and (viii) all nine patients with CBP9 cirrhosis, the most severe grade allowed into the trial, were randomly assigned to the Namodenoson treatment group (OS=3.5 months), a fact which has distorted the results in the whole population.
We believe these data strongly support the progression into Phase III.
NAFLD/NASH
We are conducting a Phase II multicenter, randomized, double-blind, placebo-controlled, dose-finding study of the efficacy and safety of Namodenoson in the treatment of NAFLD and NASH. We plan to enroll approximately 60 patients with NAFLD, with or without NASH, in three arms, including two different dosages of Namodenoson (12.5 mg and 25 mg) and a placebo, given via oral tablets twice daily.
The study’s primary endpoints are the mean percent change from baseline in ALT levels and safety. The secondary endpoint includes percent change from baseline in hepatic steatosis measured by magnetic resonance imaging determined by proton-density fat-fraction and additional metabolic parameters. In addition, an assessment of the pharmacokinetics of Namodenoson and the A3AR biomarker will be evaluated prior to treatment and its correlation to patients’ response to the drug will be analyzed upon study conclusion. Furthermore, the exploratory objective of this study is to evaluate the effects of Namodenoson on relevant biomarkers, such as adiponectin, leptin, C-reactive protein, and liver stiffness as determined by Fibroscan. The study is being conducted at the Hadassah Medical Center and Rabin Medical Center and we aim to release data in the second half of 2019.
50
Additional Developments with Namodenoson
Anti-Obesity
In January 2019, we announced new pre-clinical findings demonstrating that Namodenoson, inhibits lipid production and fat accumulation in adipocytes (lipid producing cells). More specifically, Namodenoson showed a significant decrease in lipid production and fat accumulation utilizing 3T3-L1 adipocytes, functioning as lipid producing cells and are also responsible for fat storage. Namodenoson was also shown to inhibit the proliferation of adipocytes, further hampering the expansion of fat producing cells. These findings, together with the excellent safety profile of Namodenoson, support its potential utilization as an anti-obesity drug. A patent application for the utilization of Namodenoson as an anti-obesity drug has been filed.
JC Virus
In April 2011, we announced that, in laboratory study, Namodenoson inhibited the reproduction of the JC virus, a type of polyomavirus, which is dormant in approximately 70% to 90% of the world population. However, in patients treated with biological drugs, including monoclonal antibody therapeutics, such as anti-TNFs or anti-CD20, JC virus replication may occur, resulting in development of progressive multifocal leukoencephalopathy, or PML, which is characterized by progressive damage or inflammation of the white matter of the brain and, eventually, death. The ability of Namodenoson to suppress the JC virus culture, as indicated in the laboratory study, may indicate that it may be used for the treatment of PML as a combination therapy with biological drugs. As Namodenoson is already in various stages of clinical development for other indications, its efficacy for this new application may be tested in clinical trials.
CF602
The allosteric modulator, CF602, is our third drug candidate in its pipeline. CF602 is an orally bioavailable small molecule, which enhances the affinity of the natural ligand, adenosine, to its A3AR. The advantage of this molecule is its capability to target specific areas where adenosine levels are increased. Normal body cells and tissues are refractory to allosteric modulators. This approach complements the basic platform technology of Can-Fite, utilizing the Gi coupled protein A3AR as a potent target in inflammatory diseases. CF602 has demonstrated proof of concept for anti-inflammatory activity in in vitro and in vivo studies performed by us.
During clinical studies conducted with our product candidates, other than CF602, patients suffering from sexual dysfunction reported that they returned to normal functioning following the treatment with such drugs. We believe that these findings are correlated with our platform technology, which is the targeting of the A3AR. Adenosine, like nitric oxide, is a potent and short-lived vaso-relaxant that functions via intracellular signaling (in particular, through cAMP) to promote smooth muscle relaxation. Recent studies conducted by others show that adenosine functions to relax the corpus cavernosum and thereby promote penile erection.
CF602 was tested in an experimental animal model of diabetic rats, which similar to diabetic patients, suffer from sexual dysfunction. Erectile dysfunction was assessed by monitoring the ratio between intra-cavernosal pressure, or ICP, and mean arterial pressure, or MAP, as a physiological index of erectile function. The ICP/MAP for the CF602 treated group improved by 118% over the placebo group. This data is similar to that achieved earlier by sildenafil (Viagra) in preclinical studies. In addition, treatment with CF602 reversed smooth muscle and endothelial damage, in a dose dependent manner, leading to the improvement in erectile dysfunction.
Further studies of CF602 have revealed that CF602 restores the impaired vascular endothelial growth factor system in the penis of diabetes mellitus rats, thereby inducing an increase in nitric oxide resulting in significant improvement of penile erection compared to placebo. This mechanism of action is similar to that of sildenafil, with CF602 demonstrating effects on erection superior to that demonstrated by sildenafil in animal studies. Among the most important factors to affect erectile function is nitric oxide, which is released by endothelial cells that line the corpus cavernosum and control smooth muscle relaxation and vascular inflow. It has been well established that release of nitric oxide is diminished in diabetes.
In addition, CF602 induced a dose-dependent, linear effect in a diabetic mellitus rat model after treatment with one single dose of CF602. One hour after dosing, sexual function was measured. Statistically significant full recovery from erectile dysfunction took place in rats treated with a 500 µ/kg dose.
51
According to the American Diabetes Association, approximately 30 million children and adults have diabetes mellitus in the United States. It is estimated that 35-75% of men with diabetes mellitus suffer from erectile dysfunction.
In November 2016, a Notice of Allowance was granted to us by the USPTO for our patent covering A3AR ligands for use in the treatment of erectile dysfunction. The patent addresses methods for treating erectile dysfunction with different A3AR ligands including our erectile dysfunction drug candidate, CF602. With this new broader patent protection, we made a strategic decision to investigate additional compounds, owned by us, for the most effective and safest profile in this indication. As such, we postponed our planned IND submission for this indication and are currently conducting efficacy and safety IND enabling studies with two additional compounds that belong to the family of allosteric molecules, similar to CF602, for the treatment of sexual dysfunction.
Commercial Biomarker Test
In March 2015, we completed the development of a commercial predictive biomarker blood test kit for A3AR. The biomarker test can be used at any molecular biology lab, where a small blood sample from a prospective patient would be tested and within just a few hours, results indicate if the patient would benefit from treatment with our drugs, which are currently in clinical trials for rheumatoid arthritis, psoriasis, and liver cancer.
The USPTO previously issued to us a patent for the utilization of A3AR as a biomarker to predict patient response to our drug Piclidenoson in autoimmune inflammatory indications.
In-Licensing Agreements
The following is a summary description of our in-licensing agreement with Leiden University. Our previously granted license with NIH expired in June 2015 with the expiration of certain patents. The description provided below does not purport to be complete and is qualified in its entirety by the complete agreement, which is attached as an exhibit to this Annual Report on Form 20-F.
Leiden University Agreements
On November 2, 2009, we entered into a license agreement, or the Leiden University Agreement, with Leiden University. Leiden University is affiliated with NIH and is the joint owner with NIH of the patents licensed pursuant to the Leiden University Agreement. The Leiden University Agreement grants an exclusive license for the use of the patents of several compounds, including CF602, that comprise certain allosteric compound drugs, and for the use, sale, production and distribution of products derived from such patents in the territory, i.e., China and certain countries in Europe (Austria, Belgium, Denmark, France, Germany, Italy, Spain, Sweden, Switzerland, Holland and England). Subject to certain conditions, we may sublicense the Leiden University Agreement. However, the U.S. government has an irrevocable, royalty-free, paid-up right to practice the patent rights throughout the territory on behalf of itself or any foreign government or international organization pursuant to any existing or future treaty or agreement to which the U.S. government is a signatory and the U.S. government may require us to grant sublicenses when necessary to fulfill health or safety needs.
Pursuant to the Leiden University Agreement, we are committed to make the following payments: (i) a one-time concession commission of 25,000 Euros; (ii) annual royalties of 10,000 Euros until clinical trials commence; (iii) 2% to 3% of net sales value, as defined in the Leiden University Agreement, received by us; (iv) royalties of up to 850,000 Euros based on certain progress milestones in the clinical stages of the products which are the subject of the patent under the Leiden University Agreement; and (v) if we sublicense the agreement, we will provide Leiden University royalties at a rate of 2-3% of net sales value, as defined in the Leiden University Agreement, and 10% of certain consideration received for granting the sublicense. In the event that we transfer to a transferee the aspect of our business involving the Leiden University Agreement, we must pay to Leiden University an assignment royalty of 10% of the consideration received for the transfer of the agreement. However, a merger, consolidation or any other change in ownership will not be viewed as an assignment of the agreement. In addition, we have agreed to bear all costs associated with the prosecution of the patents and patent applications to which we are granted a license under the Leiden University Agreement. As of December 31, 2018, we have paid approximately 115,000 Euros in royalties to Leiden University in connection with the Leiden University Agreement.
52
The Leiden University Agreement expires when the last of the patents expires in each country of the territory, unless earlier terminated in accordance with the terms of the Leiden University Agreement. The last of such patents is set to expire on 2027. The termination rights of the parties include, but are not limited to, (i) the non-defaulting party’s right to terminate if the defaulting party does not cure within 90 days of written notice identifying the default and requesting remedy of the same; and (ii) Leiden University’s right to terminate if we become insolvent, have a receiver appointed over our assets or initiate a winding-up. In addition, Leiden University may terminate the agreement when it is determined, in consultation with NIH, that termination is necessary to alleviate health and safety needs and certain other similar circumstances.
Out-Licensing and Distribution Agreements
The following are summary descriptions of certain out-licensing and distribution agreements to which we are a party.
Kwang Dong Agreements
On December 22, 2008, we entered into a license agreement with KD, or the Kwang Dong License Agreement, for the use, development and marketing of Piclidenoson in the Republic of Korea with respect to rheumatoid arthritis. In addition, the Kwang Dong License Agreement grants to KD an exclusive, royalty-free license to use certain of our trademarks, as determined from time to time, in connection with the distribution, marketing, promotion and sale of any products derived from Piclidenoson pursuant to the Kwang Dong License Agreement.
The Kwang Dong License Agreement also provides for the creation of a four member joint committee consisting of two members from each party for the purpose of serving as a joint source of experience and knowledge in Piclidenoson development and to facilitate communication and coordination between the parties with respect to such development. The joint committee will, among other things specifically identified in the Kwang Dong License Agreement, provide to the parties opinions, proposals, ideas and updates with respect to the Piclidenoson development processes conducted separately by each party.
According to the Kwang Dong License Agreement, we are entitled to receive or have received the following payments: (i) a non-refundable amount of $300,000 paid within 30 days of the effective date of the agreement; (ii) an amount of up to $1.2 million based on our compliance with certain milestones, including but not limited to, the conclusion of the Phase II clinical trial for Piclidenoson for treating rheumatoid arthritis and the receipt of various regulatory authorizations; and (iii) annual royalties of 7% of annual net sales of the licensed drug in the Republic of Korea. In addition to the amounts detailed above, we will be entitled to additional payments based on sales of raw materials to KD for the purpose of developing, producing and marketing Piclidenoson. To date, we have received a total of $500,000 from KD in an upfront payment.
The Kwang Dong License Agreement is effective until KD completes all payments required thereunder, unless it is earlier terminated as a result of a material breach not cured within the specified time frame, the breach by KD of the Kwang Dong Purchase Agreement (as defined below) or the initiation of bankruptcy or insolvency related proceedings.
Pursuant to a share purchase agreement entered into with KD at the same time as the Kwang Dong License Agreement, KD purchased 95,304 of our ordinary shares, representing approximately 1.0% of our share capital on a fully diluted basis, as of the date of the purchase, or the Kwang Dong Purchase Agreement. The shares were purchased for a premium of 50% on the shares’ average closing price for the ten days preceding December 11, 2008, or a purchase price of NIS 0.455 per share.
After the TASE approved such shares for the listing for trade on January 5, 2009, the shares were allocated to KD and the transaction was finalized in January 2009. To date, KD had paid us approximately $1.3 million, which represents milestone payments pursuant to the Kwang Dong License Agreement, an advance of certain amounts to become due under the Kwang Dong License Agreement and the purchase price for the shares.
53
Cipher Pharmaceuticals Agreement
On March 20, 2015, we entered into a Distribution and Supply Agreement with Cipher granting Cipher the exclusive right to distribute Piclidenoson in Canada for the treatment of psoriasis and rheumatoid arthritis.
Under the Distribution and Supply Agreement, we are entitled to CAD 1.65 million upon execution of the agreement plus milestone payments upon receipt of regulatory approval by the Therapeutic Products Directorate of Health Canada, or Health Canada, for Piclidenoson and the first delivery of commercial launch quantities as follows (i) CAD 1 million upon the first approved indication for either psoriasis or rheumatoid arthritis, and (ii) CAD 1 million upon the second approved indication for either psoriasis or rheumatoid arthritis. In addition, following regulatory approval, we shall be entitled to a royalty of 16.5% of net sales of Piclidenoson in Canada and reimbursement for the cost of manufacturing Piclidenoson. We are also entitled to a royalty payment for any authorized generic of Piclidenoson that Cipher distributes in Canada. To date, we have received a total of $1.3 million from Cipher in an upfront payment.
We are responsible for supplying Cipher with finished product for distribution and conducting product development activities while Cipher is responsible for distributing, marketing and obtaining applicable regulatory approvals in Canada. The Distribution and Supply Agreement has an initial term of fifteen years, automatically renewable for additional five-year periods and may be terminated in certain limited circumstances including certain breaches of the agreement and failure to achieve certain minimum quantities of sales during the contract period.
The timeline to regulatory submissions to Health Canada will be determined by the completion of the remaining clinical trial program.
CKD Agreement
On October 25, 2016, we entered into an exclusive Distribution Agreement with CKD for the exclusive right to distribute Namodenoson for the treatment of liver cancer in South Korea, upon receipt of regulatory approvals. On February 25, 2019, the Distribution Agreement was amended to expand the exclusive right to distribute Namodenoson for the treatment of NASH in South Korea. The Distribution Agreement further provides that we will deliver finished product to CKD and grant CKD a right of first refusal to distribute Namodenoson for other indications for which we develop Namodenoson.
The Distribution Agreement provides for up to $3,000,000 in upfront and milestone payments payable with respect to the liver cancer indication and up to $3,000,000 with respect to the NASH indication. In addition, we are entitled to a transfer price of the higher of the manufacturing cost plus 10% or 23% of net sales of Namodenoson following commercial launch in South Korea. To date, we have received a total of $1,000,000 from CKD, $500,000 in upfront payments and a further $500,000 for a milestone payment received in the third quarter of 2017 upon receipt by CKD of a positive result from the preliminary review by the MFDS on obtaining orphan drug designation in South Korea.
The Distribution Agreement has an initial term of 10 years from first commercial sale of Namodenoson for the treatment of liver cancer or NASH and is renewable for additional 3-year periods unless either party gives notice of termination at least 6 months prior to the then current term. The Distribution Agreement may be terminated by CKD upon 30 days prior written notice if we fail to successfully complete our ongoing Phase II clinical trial for Namodenoson and we may terminate the Distribution Agreement upon 30 days prior written notice if certain commercialization milestones are not met by CKD or certain minimum quantities of sales are not made during the contract period. In addition, either party may terminate the Distribution Agreement in the event of an uncured material breach or insolvency.
54
Gebro Agreement
On January 8, 2018, we entered into a Distribution and Supply Agreement with Gebro, granting Gebro the exclusive right to distribute Piclidenoson in Spain, Switzerland, Liechtenstein and Austria for the treatment of psoriasis and rheumatoid arthritis.
Under the Distribution and Supply Agreement, we are entitled to €1,500,000 upon execution of the agreement plus milestone payments upon achieving certain clinical, launch and sales milestones, as follows: (i) €300,000 upon initiation of the ACRobat Phase III clinical trial for the treatment of rheumatoid arthritis and €300,000 upon the initiation of the COMFORT Phase III clinical trial for the treatment of psoriasis, (ii) between €750,000 and €1,600,000 following first delivery of commercial launch quantities of Piclidenson for either the treatment of rheumatoid arthritis or psoriasis, and (iii) between €300,000 and up to €4,025,000 upon meeting certain net sales. In addition, following regulatory approval, we shall be entitled to double digit percentage royalties on net sales of Piclidenoson in the territories and payment for the manufacturing Piclidenoson. To date, we have received a total of €2,100,000 from Gebro in upfront and milestone payments.
We are initially responsible for supplying Gebro with finished product for distribution and obtaining EMA and Swissmedic marketing approval while Gebro is responsible for distributing, marketing and obtaining pricing and reimbursement approvals in the territories. The Distribution and Supply Agreement has an initial term of fifteen years, automatically renewable for additional five-year periods and may be terminated in certain limited circumstances including certain breaches of the agreement and failure to achieve certain minimum quantities of sales during the contract period.
CMS Medical
On August 6, 2018, we entered into a License, Collaboration and Distribution Agreement with CMS Medical, for the exclusive right to develop, manufacture and commercialize Piclidenoson for the treatment of rheumatoid arthritis and psoriasis and Namodenoson for the treatment of HCC and NAFLD/NASH in China (including Hong Kong, Macau and Taiwan).
Under the License, Collaboration and Distribution Agreement, we are entitled to $2,000,000 upon execution of the agreement plus milestone payments of up to $14,000,000 upon achieving certain regulatory milestones and payments of up to $58,500,000 upon achieving certain sales milestones, as follows: (i) $500,000 upon the granting of the marketing authorization of Piclidenoson in the United States for rheumatoid arthritis; (ii) $500,000 upon the granting of the marketing authorization of Piclidenoson in the European Union for rheumatoid arthritis; (iii) $500,000 upon the granting of the marketing authorization of Piclidenoson in the United States for psoriasis; (iv) $500,000 upon the granting of the marketing authorization of Piclidenoson in the European Union for psoriasis; (v) $500,000 upon the granting of the marketing authorization of Namodenoson in the United States for HCC; (vi) $500,000 upon the granting of the marketing authorization of Namodenoson in the European Union for HCC; (vii) $500,000 upon the granting of the marketing authorization of Namodenoson in the United States for NAFLD/NASH; (viii) $500,000 upon the granting of the marketing authorization of Namodenoson in the European Union for NAFLD/NASH; (ix) $2,500,000 upon the issuance of an imported drug license permitting the product to be imported into and marketed in China, or the IDL and granting of marketing authorization of Piclidenoson in China for rheumatoid arthritis; (x) $2,500,000 upon the issuance of the IDL and granting of marketing authorization of Piclidenoson in China for for psoriasis; (xi) $2,500,000 upon the issuance of the IDL and granting of marketing authorization of Namodenoson in China for HCC; (xii) $2,500,000 upon the issuance of the IDL and granting of marketing authorization of Namodenoson in China for NAFLD/NASH; and (xiii) between $1,000,000 and up to $30,000,000 upon meeting certain net sales. In addition, following regulatory approval, we shall be entitled to double-digit percentage royalties on net sales of Piclidenoson and Namodenoson in the licensed territories. To date, we have received a total of $2,000,000 from CMS Medical in upfront and milestone payments.
According to the agreement, CMS Medical will be responsible for the development of Piclidenoson and Namodenoson to obtain regulatory approval in China and shall be further responsible for obtaining and maintaining regulatory approval in China for the indications described above. We may, at the option of CMS Medical, supply finished product to CMS Medical.
The License, Collaboration and Distribution Agreement shall continue in force unless earlier terminated and may be terminated in certain limited circumstances including certain breaches of the agreement and failure to achieve certain minimum quantities of sales during the contract period. Following expiration of the term of the agreement, the license granted shall become non-exclusive, fully paid, royalty free and irrevocable.
55
SKK Agreement
On August 27, 2015, we entered into an agreement with Japan-based Seikagaku Corporation, or SKK, terminating its license agreement with us. SKK informed us that it is strategically focused on expanding its core research and development activities in the field of glyco-science. Under the license agreement, SKK was granted a license for the use, development and marketing of Piclidenoson in Japan with respect to inflammatory indications, except for ophthalmic disease indications. The termination agreement provides, among other things, that all licenses and rights granted to SKK terminate and all clinical and non-clinical studies conducted by SKK shall be transferred free of charge to us. Over the life of the license, we received an aggregate of approximately $8.5 million from SKK.
Total Revenues by Category of Activity and Geographic Markets
Historically, we have generated revenues from payments received pursuant to our out-licensing agreements with Gebro, Cipher, KD, CMS Medical and SKK with respect to Piclidenoson and CKD with respect to Namodenoson. See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”. We recorded revenues of $2 million for the year ended December 31, 2018 as a result from advance payment received in August 2018 under the distribution agreement with CMS Medical. We recorded revenues of $1.3 million for the year ended December 31, 2018 as a result from recognition a portion of an advance payment received in January 2018 under the distribution agreement with Gebro. We recorded revenues of $0.1 million for the year ended December 31, 2018 under the Distribution Agreement with CKD which was due to the recognition of a portion of the $0.5 million advance payment received in December 2016 under the Distribution Agreement with CKD. We recorded revenues of $0.6 million for the year ended December 31, 2017 under the Distribution Agreement with CKD which was due to the recognition of a portion of the $0.5 million advance payment received in December 2016 under the Distribution Agreement with CKD and a payment of $0.5 million as a result of a milestone achievement. We recorded revenues of $0.4 million for the year ended December 31, 2018 and $0.2 million for the year ended December 31, 2017 which was due to the recognition of a portion of the CAD 1.65 million advance payment received in March 2015 under the Distribution and Supply Agreement with Cipher. We expect to generate future revenues through our current and potential future out-licensing arrangements with respect to Piclidenoson and Namodenoson based on the progress we make in our clinical trials.
Seasonality
Our business and operations are generally not affected by seasonal fluctuations or factors.
Raw Materials and Suppliers
We believe that the raw materials that we require to manufacture Piclidenoson, Namodenoson and CF602 are widely available from numerous suppliers and are generally considered to be generic industrial chemical supplies. We do not rely on a single or unique supplier for the current production of any therapeutic small molecule in our pipeline.
Manufacturing
We are currently manufacturing our API through a leading CRO. The relevant suppliers of our drug products are compliant with both current Good Manufacturing Practices, or cGMP, and current Good Laboratory Practices, or cGLP, and allow us to manufacture drug products for our current clinical trials. We anticipate that we will continue to rely on third parties to produce our drug products for clinical trials and commercialization.
There can be no assurance that our drug candidates, if approved, can be manufactured in sufficient commercial quantities, in compliance with regulatory requirements and at an acceptable cost. We and our contract manufacturers are, and will be, subject to extensive governmental regulation in connection with the manufacture of any pharmaceutical products or medical devices. We and our contract manufacturers must ensure that all of the processes, methods and equipment are compliant with cGMP for drugs on an ongoing basis, as mandated by the FDA and other regulatory authorities, and conduct extensive audits of vendors, contract laboratories and suppliers.
56
Contract Research Organizations
We outsource certain preclinical and clinical development activities to CROs, which in pre-clinical studies work according to cGMP and cGLP. We believe our clinical CROs comply with guidelines from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, which attempt to harmonize the FDA and the EMA regulations and guidelines. We create and implement the drug development plans and, during the preclinical and clinical phases of development, manage the CROs according to the specific requirements of the drug candidate under development.
Marketing and Sales
We do not currently have any marketing or sales capabilities. We intend to license to, or enter into strategic alliances with, larger companies in the pharmaceutical business, which are equipped to market and/or sell our products, if any, through their well-developed marketing capabilities and distribution networks. We intend to out-license some or all of our worldwide patent rights to more than one party to achieve the fullest development, marketing and distribution of any products we develop.
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for our product candidates, technology and know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions and improvements that we believe are important to the development of our business. We also rely on trade secrets, know-how and continuing technological innovation to develop and maintain our proprietary position.
Patents
As of March 21, 2019, we owned or exclusively licensed (from Leiden University) 13 patent families that, collectively, contain approximately 188 issued patents and pending patent applications in various countries around the world relating to our two clinical candidates, Piclidenoson and Namodenoson, and our preclinical candidate, CF602. Patents related to our drug candidates may provide future competitive advantages by providing exclusivity related to the composition of matter, formulation and method of administration of the applicable compounds and could materially improve their value. The patent positions for our leading drug candidates are described below.
With respect to our product candidates, we currently own patents and/or have patent applications pending in several countries around the world for the following families of patents:
| ● | A3AR ligands to treat proliferative diseases (inflammation/cancer) - a family of patents which pertains to the use of substances that bind to the A3AR, including Piclidenoson and Namodenoson; the pharmaceutical uses to which such family relates include the treatment of proliferative diseases, such as cancer, psoriasis and autoimmune diseases. Such patents were granted in the United States, Europe (by the European Patent Office, or the EPO, and validated in Austria, Belgium, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Luxembourg, the Netherlands, Poland, Portugal, Spain, Sweden, Switzerland, and the United Kingdom), Australia, Canada, Israel, China, Japan, South Korea, Mexico, Russia and Hong Kong. These patents are set to expire in 2020, other than the U.S. patent that will expire in 2022; |
| ● | A3AR ligands to treat viral diseases - a family of patents and a patent application which pertain to use of substances that bind to the A3AR for the treatment of viral diseases, such as AIDS and hepatitis, and which inhibit viral replication. Such patents were granted in the United States, in Europe (by the EPO and validated in France, Germany, Italy, Switzerland and the United Kingdom), Australia, China, Israel, Japan, Singapore, Canada and Hong Kong. These patents have a filing date of January 1, 2002 and a priority date of January 16, 2001 and are set to expire in 2022, other than the U.S. patent that will expire in 2023; |
57
| ● | A3AR ligands to treat RA - a patent which pertains to the use of A3AR agonists for the treatment of inflammatory arthritis, in particular rheumatoid arthritis. This patent was granted in the United States and is set to expire in 2023; |
| ● | A3AR as a predictive and follow up biomarker - a family of patents and patent applications which pertain to a method of identifying inflammation, determining its severity, and determining and monitoring the efficacy of the anti-inflammatory treatment by determining the level of A3AR expression in white blood cells as a biological marker for inflammation. These patents were granted in certain countries in the United States, Europe (by the EPO and validated in France, Germany, Italy, Spain, Switzerland and the United Kingdom), Australia, Israel, Japan, China, Mexico and Canada. The patents are set to expire in 2025. There is a patent application pending in Brazil. Each of the patents and the patent application has a filing date of November 30, 2005 and a priority date of December 2, 2004; |
| ● | Specific dose to protect psoriasis - a family of patents and patent applications which pertains to the use of a specific dose level of Piclidenoson (total daily dose of 4.0 mg) for the treatment of psoriasis. Such a patent was granted in Israel, Japan, the United States, South Korea and Europe (by the EPO and validated in in Austria, Belgium, Denmark, France, Germany, Italy, the Netherlands, Spain, Sweden, Switzerland and the United Kingdom). The patent is set to expire in 2030. The patent applications are pending in the China, Hong Kong, and India each with a filing date of September 6, 2010 and a priority date of September 6, 2009; | |
| ● | Piclidenoson method of synthesis - a family of patents and patent applications which pertain to the method for producing Piclidenoson. Such patents were granted in the United States, India, China, Japan, Israel and Europe (by the EPO and validated in in Austria, Belgium, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Luxembourg, Monaco, the Netherlands, Norway, Poland, Portugal, Spain, Sweden, Switzerland, Turkey and the United Kingdom. These patents are set to expire in 2028. Each patent and patent application has a filing date of March 13, 2008 and a priority date of March 14, 2007; | |
| ● | Osteoarthritis indication - a family of patents and patent applications which pertain to the use of A3AR agonists for the treatment of OA. Such patents were granted in Europe (by the EPO and validated in Austria, Belgium, Denmark, France, Germany, Italy, Spain, Sweden, Switzerland, Netherlands and the United Kingdom), Australia, Canada, South Korea, China, Israel, Japan and Mexico. The patents are set to expire in 2026. Patent applications are pending in the United States and Brazil with the United States application being recently allowed (issue fee due Apr 03, 2019). These patents and patent applications have a filing date of November 29, 2006 and a priority date of November 30, 2005; | |
| ● | Liver protection - a family of patents and patent applications which pertains to the use of A3AR agonists for increasing liver cell division, intended to induce liver regeneration following injury or surgery. Such patents were granted in China, Israel, Japan, USA and Europe (by the EPO and validated in Austria, Belgium, Denmark, France, Germany, Italy, the Netherlands, Poland, Spain, Sweden, Switzerland, United Kingdom and Turkey). Each patent in this family has a filing date of October 22, 2007 and a priority date of October 15, 2007; | |
| ● | Sexual dysfunction - a family of patent applications which pertain to treatment of sexual dysfunction. This family includes granted patents in the United States, Australia, China, Hong Kong, and Japan and patent applications in Israel, Canada, Europe, Mexico, and South Korea with the applications in Europe and South Korea being recently allowed. The patent applications have a filing date of August 8, 2013 with priority dates of August 8, 2012 and November 12, 2012; |
58
| ● | CAR T induced cytokine release syndrome - a family of patent applications which pertains to the use of A3AR ligands for managing cytokine release syndrome. This family includes a patent application in Israel and a PCT claiming priority from this Israeli application. The PCT application has a filing date of September 16, 2018 and the Israeli patent application has a filing date of September 17, 2017 and | |
| ● | NAFLD/NASH - a family of patent applications which pertain to the use of A3AR ligands for treatment of ectopic fat accumulation. This family includes patent applications in Israel, China, Europe, USA, Brazil, Canada, Japan, Mexico and South Korea. The patent applications have a filing date of November 22, 2016. | |
| ● | Obesity - a patent application in Israel which pertains to the use of A3AR ligand for reducing level of adipocytes. This patent application has a filing date of January 6, 2019 and will serve as a priority document to an International PCT application due to be filed no later than January 6, 2020. |
We currently hold an exclusive license from Leiden University of the Netherlands to a family of patents and patent applications that relate to the allosteric modulators of the A3AR, which includes the allosteric modulator CF602. This exclusive license relates to patents that were granted in the United States, China, Japan, South Korea, India and in Europe (validated in, Austria, Belgium, Denmark, France, Germany, Italy, the Netherlands, Spain, Sweden, Switzerland and United Kingdom). These granted patents are set to expire in 2027.
We believe that our owned and licensed patents provide broad and comprehensive coverage of our technology, and we intend to aggressively enforce our intellectual property rights if necessary to preserve such rights and to gain the benefit of our investment. However, as a result of the termination of the NIH license agreement between Can-Fite and NIH in June 2015 due to patent expiration, we no longer hold rights to a family of composition of matter patents relating to Piclidenoson that were licensed from NIH. Nevertheless, because Piclidenoson may be a NCE following approval of an NDA, we, if we are the first applicant to obtain NDA approval, may be entitled to five years of data exclusivity in the United States with respect to such NCEs. Analogous data and market exclusivity provisions, of varying duration, may be available in Europe and other foreign jurisdictions. We may also be entitled to the rights under Can-Fite’s pharmaceutical use issued patents with respect to Piclidenoson, which provide patent exclusivity within the ophthalmic field until the mid-2020s. While we believe that we may be able to protect our exclusivity in the ophthalmic field through such use patent portfolio and such period of exclusivity, the lack of composition of matter patent protection may diminish our ability to maintain a proprietary position for our intended uses of Piclidenoson. Moreover, we cannot be certain that we will be the first applicant to obtain an FDA approval for any indication of Piclidenoson and we cannot be certain that we will be entitled to NCE exclusivity. In addition, we have discontinued the prosecution of a family of pending patent applications under joint ownership of Can-Fite and NIH pertaining to the use of A3AR agonists for the treatment of uveitis. Such diminution of our proprietary position could have a material adverse effect on our business, results of operation and financial condition.
The patent positions of companies like ours are generally uncertain and involve complex legal and factual questions. Our ability to maintain and solidify our proprietary position for our technology will depend on our success in obtaining effective claims and enforcing those claims once granted. We do not know whether any of our patent applications or those patent applications that we license will result in the issuance of any patents. Our issued patents and those that may issue in the future, or those licensed to us, may be challenged, narrowed, circumvented or found to be invalid or unenforceable, which could limit our ability to stop competitors from marketing related products or the length of term of patent protection that we may have for our products. Neither we nor our licensors can be certain that we were the first to invent the inventions claimed in our owned or licensed patents or patent applications. In addition, our competitors may independently develop similar technologies or duplicate any technology developed by us, and the rights granted under any issued patents may not provide us with any meaningful competitive advantages against these competitors. Furthermore, because of the extensive time required for development, testing and regulatory review of a potential product, before any of our products can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of the patent.
59
Trade Secrets
We may rely, in some circumstances, on trade secrets to protect our technology. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements and assignment of inventions agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, such agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors or others.
Scientific Advisory Board
We seek advice from our Scientific Advisory Board on scientific and medical matters generally. We call for Scientific Advisory Board meetings on an as-needed basis. The following table sets forth certain information with respect to our Scientific Advisory Board member.
| Name | Position/Institutional Affiliation | |
| Nabil Hanna, Ph.D. | Former Chief Science Officer of Biogen-Idec |
Clinical Advisory Board
Our Clinical Advisory Board, which consists of six members, a leading U.S.-based rheumatologist, oncologist, dermatologist, and three hepatologists, who play an active role in consulting with us with respect to clinical drug development. We call for Clinical Advisory Board meetings on an as-needed basis. The following table sets forth certain information with respect to our Clinical Advisory Board members.
| Name | Position/Institutional Affiliation | |
| Dr. Michael Weinblatt | Head, Division of Rheumatology, Immunology and Allergy, Brigham and Women’s Hospital | |
| Dr. Keith Stuart | Chairman, Department of Hematology and Oncology; Professor of Medicine, Tufts University School of Medicine; Lahey Clinic Medical Center | |
| Dr. Jonathan Wilkin | Former Head, Dermatology Division, FDA | |
| Dr. Scott Friedman | Dean for Therapeutic Discovery and Chief of the Division of Liver Diseases at the Icahn School of Medicine at Mount Sinai in New York | |
| Dr. Arun Sanyal | Professor of Medicine, Physiology and Molecular Pathology at Virginia Commonwealth University School of Medicine | |
| Dr. Rifaat Safadi | Head of the Liver Unit, Gastroenterology and Liver Diseases, Division of Medicine at Hadassah Medical Center and Professor of Internal Medicine, Bowel, Liver Disease, and Metabolic Syndrome at Hadassah University in Israel |
60
Competition
The pharmaceutical industry is characterized by rapidly evolving technology, intense competition and a highly risky, costly and lengthy research and development process. Adequate protection of intellectual property, successful product development, adequate funding and retention of skilled, experienced and professional personnel are among the many factors critical to success in the pharmaceutical industry.
Our technology platform is based on the finding that the A3AR is highly expressed in pathological cells, such as various tumor cell types and inflammatory cells. We believe that targeting the A3AR with synthetic and highly selective A3AR agonists, such as Piclidenoson and Namodenoson, and allosteric modulators, such as CF602, induces anti-cancer and anti-inflammatory effects. Currently, our drug candidates, Piclidenoson, Namodenoson and CF602 are being developed to treat autoimmune inflammatory indications, oncology and liver diseases as well as sexual dysfunction, including but not limited to psoriasis, rheumatoid arthritis, HCC and NASH. Preclinical studies have also indicated that our drug candidates have the potential to treat additional inflammatory diseases, such as sexual dysfunction, Crohn’s disease, oncological diseases and viral diseases, such as the JC virus, and obesity.
Despite the competition, however, we believe that our drug candidates have unique characteristics and advantages over certain drugs currently available on the market and under development to treat these indications. We believe that our pipeline of drug candidates has exhibited a potential for therapeutic success with respect to the treatment of autoimmune-inflammatory, oncological and liver diseases. We believe that targeting the A3AR with synthetic and highly selective A3AR agonists, such as Piclidenoson and Namodenoson, and allosteric modulators, such as CF602, induces anti-cancer and anti-inflammatory effects.
We believe the characteristics of Piclidenoson, as exhibited in our clinical studies to date, including its good safety profile, clinical activity, simple and less frequent delivery through oral administration and its low cost of production, position it well against the competition in the autoimmune-inflammatory markets, including the psoriasis and rheumatoid arthritis markets, where treatments, when available, often include injectable drugs, many of which can be highly toxic, expensive and not always effective. For example, while TNF inhibitor therapies transformed the treatment for many patients, a substantial percentage of patients (40% to 60%) do not respond to either a DMARD or biologic therapies (Simsek, 2010).
Pre-clinical pharmacology studies in different experimental animal models of arthritis revealed that Piclidenoson acts as a DMARD, which, when coupled with its good safety profile, makes it competitive in the psoriasis, rheumatoid arthritis and OA markets. Our recent findings also demonstrate that a biological predictive marker can be utilized prior to treatment with Piclidenoson, which may allow it to be used as a personalized medicine therapeutic approach for the treatment of rheumatoid arthritis, potentially leading to an improvement in response rate for patients. Like Piclidenoson, Namodenoson has a good safety profile, is orally administered and has a low cost of production, which we believe positions it well in the HCC market, where only one drug, Nexavar (sorafenib), has been approved by the FDA.
In addition, our human clinical data suggests that A3AR may be a biological marker in that high A3AR expression prior to treatment has been predictive of good patient response to our drug treatment. In fact, as a result of our research we have developed a simple blood assay to test for A3AR expression as a predictive biological marker. We hold a patent with respect to the intellectual property related to such assay and are currently utilizing this assay in our ongoing Phase IIb study of Piclidenoson for the treatment of rheumatoid arthritis.
On the other hand, other drugs on the market, new drugs under development (including drugs that are in more advanced stages of development in comparison to our drug pipeline) and additional drugs that were originally intended for other purposes, but were found effective for purposes targeted by us, may all be competitive to the current drug candidates in our pipeline. In fact, some of these drugs are well established and accepted among patients and physicians in their respective markets, are orally bioavailable, can be efficiently produced and marketed, and are relatively safe. Moreover, other companies of various sizes engage in activities similar to ours. Most, if not all, of our competitors have substantially greater financial and other resources available to them. Competitors include companies with marketed products and/or an advanced research and development pipeline. The major competitors in the arthritis and psoriasis therapeutic field include Amgen, J&J, Pfizer, Novartis, Abbvie, Celgene, Eli Lilly, Bristol-Myers, and more. Competitors in the HCC field include companies such as Bayer, Exelixis, Merck, and Bristol-Myers. Competitors in the NASH field include companies such as Gilead, Genfit, Galmed, Allergan, Intercept, and Madrigal. Competitors in the erectile dysfunction field include Pfizer, Eli Lilly and Bayer.
61
Moreover, several companies have reported the commencement of research projects related to the A3AR. Such companies include CV Therapeutics Inc. (which was acquired by Gilead), King Pharmaceuticals R&D Inv. (which was acquired by Pfizer), Hoechst Marion Roussel Inc., Novo Nordisk A/S and Inotek Pharmaceuticals. However, to the best of our knowledge, there is no approved drug currently on the market which is similar to our A3AR agonists, nor are we aware of any allosteric modulator in the A3AR product pipeline similar to our allosteric modulator with respect to chemical profile and mechanism of action.
Piclidenoson for the Treatment of Psoriasis
Psoriasis is a skin condition that affects 2% to 3% of the general population according to the National Psoriasis Foundation. The disease is manifested by scaly plaques on the skin and in the severe form has a major effect on the physical and emotional well-being of the patients. Topical agents are typically used for mild disease, phototherapy for moderate disease, and systemic agents for severe disease. For moderate to severe cases, systemic biologic drugs, delivered via intravenous injection, or IV, have dominated the market. According to the National Psoriasis Foundation, common side effects of biologics include respiratory infections, flu-like symptoms, and injection site reactions while rare side effects include serious nervous system disorders, such as multiple sclerosis, seizures, or inflammation of the nerves of the eyes, blood disorders, and certain types of cancer. We believe a significant need remains for novel oral and safe drugs for patients who do not respond to existing therapies or for whom these therapies are unsuitable.
The psoriasis therapeutic market is dominated by biological drugs that are primarily administered via IV and have potential side effects. Recently, a new oral small molecule inhibitor of phosphodiesterase 4, Celgene’s Otezla, has gained sizable market share as a result in part due to its convenience of oral dose and comparable efficacy to the biologic drugs. In January 2015, the FDA approved Cosentyx (secukinumab) by Novartis. In March 2016, the FDA approved Taltz (ixekizumab) by Eli Lilly. The psoriasis drug market is forecast to grow to $11.4 billion in 2020, according to estimates of Visiongain.
The current common treatments for psoriasis include topical and systemic drugs, steroids, immunosuppressive drugs such as Cyclosporine A by Novartis, MTX and biological drugs. Biological drugs, such as Enbrel (etanercept) by Amgen and Pfizer, Remicade (infliximab) by Centocor, Humira (adalimumab) by Abbvie, Stelara (ustekinumab) by Janssen, Otezla (aprelimast) by Celgene, Cosentyx (secukinumab) by Novartis and Taltz (ixekizumab) by Eli Lilly have significant side effects, are expensive and patients are often not responsive. For example, some of these drugs have received an FDA “black box” warning for increased risk of cancer in children and adolescents and risk of infection with Legionella and Listeria bacteria.
Many of the current rheumatoid arthritis drugs on the market or in development are also used for the treatment of psoriasis. See “Item 4. Information on the Company—B. Business Overview—Piclidenoson for the Treatment of Rheumatoid Arthritis.” In addition, several therapies are in advanced clinical development for psoriasis and many others are in Phase II or earlier stages of development.
Piclidenoson for the Treatment of Rheumatoid Arthritis
Rheumatoid arthritis is a severe disease that attacks approximately 0.6% of the U.S. population, mainly women and, in particular, postmenopausal women. According to Visiongain, the world rheumatoid arthritis market size is predicted to generate revenues of $47 billion by 2024.
Many drugs are used to treat rheumatoid arthritis, including DMARDs. These include MTX, plaquenil, sulfasalazine and leflunomide, all of which are small molecule drugs with mild effectiveness. MTX is the most commonly administered DMARD for rheumatoid arthritis. It is a generic chemotherapeutic agent marketed by several manufacturers that is administered orally. Due to its relatively toxic nature, however, MTX may result in severe side effects including sores, anemia, diarrhea, nausea/vomiting, abdominal pain, bruising/bleeding, and liver problems.
62
The second class of DMARD includes biological drugs, such as Enbrel (etanercept) by Amgen, Remicade (infliximab) by Centocor, and Humira (adalimumab) by Abbvie. These drugs are usually administered in combination with MTX and are more effective in combination, but may have severe side effects, including risk of lymphoma and serious infection. Biological drugs are administered through injection, are generally expensive and there is no biomarker to predict the response, if any. As such, response rates typically range between 40-60% (Simsek, 2010). Steroidal drugs are also used to reduce the general activity of the immune system and for pain relief. In addition, the FDA recently approved Pfizer’s Xeljanz (tofacitinib) small molecule drug, which is the first JAK inhibitor drug, or a drug that inhibits the effect of one or more of the enzymes in the janus kinase family, or a family enzymes that transfer cytokine-mediated signals, to treat rheumatoid arthritis. Moreover, several therapies, including biological drugs and small molecule drugs, are in advanced clinical development for rheumatoid arthritis including baricitninib by Eli Lilly which is pending FDA approval, while others are in Phase II or earlier stages of development.
Namodenoson for the Treatment of HCC
According to the American Cancer Society, HCC is the fifth most common form of cancer death in the U.S., the most common form of liver cancer in adults and the third most common cause of cancer-related mortality worldwide, particularly in Asia. According to the American Cancer Society, more than 700,000 people are diagnosed with liver cancer each year throughout the world and more than 600,000 persons die from liver cancer each year. Nexavar (sorafenib) by Bayer is the only approved drug for HCC and prolongs patient survival time by only a few months. According to Grand View Research, the HCC drug market is expected to reach $1.5 billion by 2022.
Several therapies are in advanced clinical development for HCC. Some drugs under development act as a single agent and some act in combination with Nexavar or approved checkpoint inhibitors pembrolizumab and/or nivolumab. Moreover, some are first line treatments while others are second line treatments. In addition, many existing approaches are used in the treatment of unresectable liver cancer, including alcohol injection, radiofrequency ablation, chemoembolization, cryoablation and radiation therapy.
Namodenoson for the Treatment of NASH
Rates of NAFLD and NASH are increasing in the United States in concert with increasing rates of obesity and diabetes. In fact, NASH is now the third leading cause of liver transplant in the United States. It is estimated that 17-33% of Americans have fatty liver, with approximately one-third going on to develop NASH. NASH is believed to affect 2-5% of adult Americans. Despite the progression of several interesting clinical-stage candidates by companies such as Gilead, Genfit, Madrigal, Conatus, Galmed, Allergan and Intercept as well as others, there are currently no FDA approved treatment options for NASH. In February 2019, Intercept announced positive topline results in its pivotal Phase 3 results of its NASH drug and as a consequence announced that it intends to file for regulatory approval in the U.S. and Europe in the second half of 2019.
By 2025, Deutsche Bank estimates the addressable pharmaceutical market for NASH will reach $35-40 billion in size.
CF602 for the Treatment of Erectile Dysfunction
According to the Massachusetts Male Aging Study in 1994, 52% of the respondents between the ages of 40 and 70 years old reported some degree of erectile dysfunction.
The most popular class of drug to treat erectile dysfunction is the phosphodiesterase type 5, or PDE5, inhibitors. These drugs block the degradative action of cyclic guanosine monophosphate, or GMP, specific PDE5 on cyclic GMP in the smooth muscle cells lining the blood vessels supplying the corpus cavernosum of the penis. An erection is caused by increased blood flow into the penis resulting from the relaxation of penile arteries and corpus cavernosal smooth muscle. This response is mediated by the release of nitric oxide from nerve terminals and endothelial cells, which stimulates the synthesis of cyclic GMP in smooth muscle cells. The inhibition of PDE5 enhances erectile function by increasing the concentration of cyclic GMP in the corpus cavernosum and pulmonary arteries.
63
Unfortunately, the systemic side effects of PDE5 inhibitors include a decrease in sitting blood pressure. This has resulted in warnings and precautions and contraindications of use in patients already taking antihypertensive agents like nitrates or alpha-blockers. A study published in the American Journal of Medicine (Selvin E., et al., 2007) found that persons with a history of heart disease, hypertension, and diabetes had a higher probability of impotence. A second study published in the same journal (Shah NP., et al, 2015) notes that vascular erectile dysfunction is a powerful marker of increased cardiovascular risk. We believe a significant market opportunity exists targeting erectile dysfunction patients contraindicated for use of the market leading products, Viagra and Cialis.
Grand View Research Inc. estimates the value of the erectile dysfunction therapeutic market to be approximately $3.2 billion by 2022.
Insurance
We maintain insurance for our offices and laboratory in Petah-Tikva, Israel. Our insurance program covers approximately $0.375 million of equipment and lease improvements against risk of loss, excluding damage from inventory theft. In addition, we maintain the following insurance: employer liability with coverage of approximately $5.0 million; third party liability with coverage of approximately $0.75 million; fire insurance coverage of approximately $0.725 million; natural disaster coverage of approximately $1.1 million; all risk coverage of approximately $0.02 million for electronic equipment and machinery insurance for laboratory refrigerators; and directors’ and officers’ liability insurance with coverage of $20.0 million per claim and $20.0 million in the aggregate and also D&O Side A DIC insurance with coverage of $5.0 million per claim and in the aggregate.
We also maintain worldwide product and clinical trial liability insurance with coverage of approximately $5 million with respect to the Piclidenoson and Namodenoson drugs used in clinical trials. We also procure additional insurance for each specific clinical trial which covers a certain number of trial participants and which varies based on the particular clinical trial. Certain of such policies are based on the Declaration of Helsinki, which is a set of ethical principles regarding human experimentation developed for the medical community by the World Medical Association, and certain protocols of the Israeli Ministry of Health.
We procure cargo marine coverage when we ship substances for our clinical studies. Such insurance is custom-fit to the special requirements of the applicable shipment, such as temperature and/or climate sensitivity. If required, we insure the substances to the extent they are stored in central depots and at clinical sites.
We believe that our insurance policies are adequate and customary for a business of our kind. However, because of the nature of our business, we cannot assure you that we will be able to maintain insurance on a commercially reasonable basis or at all, or that any future claims will not exceed our insurance coverage.
Environmental Matters
We are subject to various environmental, health and safety laws and regulations, including those governing air emissions, water and wastewater discharges, noise emissions, the use, management and disposal of hazardous, radioactive and biological materials and wastes and the cleanup of contaminated sites. We believe that our business, operations and facilities are being operated in compliance in all material respects with applicable environmental and health and safety laws and regulations. Our laboratory personnel in Israel have ongoing communication with the Israeli Ministry of Environmental Protection in order to verify compliance with relevant instructions and regulations. In addition, all of our laboratory personnel participate in instruction on the proper handling of chemicals, including hazardous substances before commencing employment, and during the course of their employment with us. In addition, all information with respect to any chemical substance that we use is filed and stored as a Material Safety Data Sheet, as required by applicable environmental regulations. Based on information currently available to us, we do not expect environmental costs and contingencies to have a material adverse effect on us. The operation of our testing facilities, however, entails risks in these areas. Significant expenditures could be required in the future if these facilities are required to comply with new or more stringent environmental or health and safety laws, regulations or requirements. See “Item 4. Information on the Company—B. Business Overview—Government Regulation and Funding—Israel Ministry of the Environment—Toxin Permit.”
64
Government Regulation and Funding
We operate in a highly controlled regulatory environment. Stringent regulations establish requirements relating to analytical, toxicological and clinical standards and protocols in respect of the testing of pharmaceuticals. Regulations also cover research, development, manufacturing and reporting procedures, both pre- and post-approval. In many markets, especially in Europe, marketing and pricing strategies are subject to national legislation or administrative practices that include requirements to demonstrate not only the quality, safety and efficacy of a new product, but also its cost-effectiveness relating to other treatment options. Failure to comply with regulations can result in stringent sanctions, including product recalls, withdrawal of approvals, seizure of products and criminal prosecution.
Before obtaining regulatory approvals for the commercial sale of our product candidates, we must demonstrate through preclinical studies and clinical trials that our product candidates are safe and effective. Historically, the results from preclinical studies and early clinical trials often have not accurately predicted results of later clinical trials. In addition, a number of pharmaceutical products have shown promising results in clinical trials but subsequently failed to establish sufficient safety and efficacy results to obtain necessary regulatory approvals. We have incurred, and will continue to incur substantial expense for and devote a significant amount of time to, preclinical studies and clinical trials. Many factors can delay the commencement and rate of completion of clinical trials, including the inability to recruit patients at the expected rate, the inability to follow patients adequately after treatment, the failure to manufacture sufficient quantities of materials used for clinical trials, and the emergence of unforeseen safety issues and governmental and regulatory delays. If a product candidate fails to demonstrate safety and efficacy in clinical trials, this failure may delay development of other product candidates and hinder our ability to conduct related preclinical studies and clinical trials. Additionally, as a result of these failures, we may also be unable to obtain additional financing.
Governmental authorities in all major markets require that a new pharmaceutical product be approved or exempted from approval before it is marketed, and have established high standards for technical appraisal which can result in an expensive and lengthy approval process. The time to obtain approval varies by country and some products are never approved. The lengthy process of conducting clinical trials, seeking approval and subsequent compliance with applicable statutes and regulations, if approval is obtained, are very costly and require the expenditure of substantial resources.
A summary of the United States, European Union and Israeli regulatory processes follow below.
United States
In the United States, the Public Health Service Act and the Federal Food, Drug, and Cosmetic Act (FDCA), as amended, and the regulations promulgated thereunder, and other federal and state statutes and regulations govern, among other things, the safety and effectiveness standards for our products and the raw materials and components used in the production of, testing, manufacture, labeling, storage, record keeping, approval, advertising and promotion of our products on a product-by-product basis.
Preclinical tests include in vitro and in vivo evaluation of the product candidate, its chemistry, formulation and stability, and animal studies to assess potential safety and efficacy. Certain preclinical tests must be conducted in compliance with good laboratory practice regulations. Violations of these regulations can, in some cases, lead to invalidation of the studies, requiring them to be replicated. After laboratory analysis and preclinical testing, a sponsor files an IND to begin human testing. Typically, a manufacturer conducts a three-phase human clinical testing program which itself is subject to numerous laws and regulatory requirements, including adequate monitoring, reporting, record keeping and informed consent. In Phase I, small clinical trials are conducted to determine the safety and proper dose ranges of our product candidates. In Phase II, clinical trials are conducted to assess safety and gain preliminary evidence of the efficacy of our product candidates. In Phase III, clinical trials are conducted to provide sufficient data for the statistically valid evidence of safety and efficacy. The time and expense required for us to perform this clinical testing can vary and is substantial. We cannot be certain that we will successfully complete Phase I, Phase II or Phase III testing of our product candidates within any specific time period, if at all. Furthermore, the FDA, the Institutional Review Board responsible for approving and monitoring the clinical trials at a given site, the Data Safety Monitoring Board, where one is used, or we may suspend the clinical trials at any time on various grounds, including a finding that subjects or patients are exposed to unacceptable health risk.
65
If the clinical data from these clinical trials (Phases I, II and III) are deemed to support the safety and effectiveness of the candidate product for its intended use, then we may proceed to seek to file with the FDA an NDA seeking approval to market a new drug for one or more specified intended uses. We have not completed our clinical trials for any candidate product for any intended use and therefore, we cannot ascertain whether the clinical data will support and justify filing an NDA. Nevertheless, if and when we are able to ascertain that the clinical data supports and justifies filing an NDA, we intend to make such appropriate filings.
The purpose of the NDA is to provide the FDA with sufficient information so that it can assess whether it ought to approve the candidate product for marketing for specific intended uses. The fact that the FDA has designated a drug as an orphan drug for a particular intended use does not mean that the drug has been approved for marketing. Only after an NDA has been approved by the FDA is marketing appropriate. A request for orphan drug status must be filed before the NDA is filed. The orphan drug designation, though, provides certain benefits, including a seven-year period of market exclusivity subject to certain exceptions. In February 2012, the FDA granted an orphan drug status for the active moiety, or the part of the drug that is responsible for the physiological or pharmacological action of the drug substance, of Namodenoson for the treatment of HCC. Subsequently, in October 2015, the EMA granted Namodenoson orphan drug designation for the treatment of HCC. See “Item 4. Information on the Company—B. Business Overview—Namodenoson”.
The NDA normally contains, among other things, sections describing the chemistry, manufacturing, and controls, non-clinical pharmacology and toxicology, human pharmacokinetics and bioavailability, microbiology, the results of the clinical trials, and the proposed labeling which contains, among other things, the intended uses of the candidate product.
We cannot take any action to market any new drug or biologic product in the United States until our appropriate marketing application has been approved by the FDA. The FDA has substantial discretion over the approval process and may disagree with our interpretation of the data submitted. The process may be significantly extended by requests for additional information or clarification regarding information already provided. As part of this review, the FDA may refer the application to an appropriate advisory committee, typically a panel of clinicians. Satisfaction of these and other regulatory requirements typically takes several years, and the actual time required may vary substantially based upon the type, complexity and novelty of the product. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly procedures on our activities. We cannot be certain that the FDA or other regulatory agencies will approve any of our products on a timely basis, if at all. Success in preclinical or early stage clinical trials does not assure success in later-stage clinical trials. Even if a product receives regulatory approval, the approval may be significantly limited to specific indications or uses and these limitations may adversely affect the commercial viability of the product. Delays in obtaining, or failures to obtain regulatory approvals, would have a material adverse effect on our business.
Even after we obtain FDA approval, we may be required to conduct further clinical trials (i.e., Phase IV trials) and provide additional data on safety and effectiveness. We are also required to gain separate approval for the use of an approved product as a treatment for indications other than those initially approved. In addition, side effects or adverse events that are reported during clinical trials can delay, impede or prevent marketing approval. Similarly, adverse events that are reported after marketing approval can result in additional limitations being placed on the product’s use and, potentially, withdrawal of the product from the market. Any adverse event, either before or after marketing approval, can result in product liability claims against us.
66
As an alternate path for FDA approval of new indications or new formulations of previously-approved products, a company may file a Section 505(b)(2) NDA, instead of a “stand-alone” or “full” NDA. Section 505(b)(2) of the FDCA, was enacted as part of the Drug Price Competition and Patent Term Restoration Act of 1984, otherwise known as the Hatch-Waxman Amendments. Section 505(b)(2) permits the submission of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. Some examples of products that may be allowed to follow a 505(b)(2) path to approval are drugs that have a new dosage form, strength, route of administration, formulation or indication. The Hatch-Waxman Amendments permit the applicant to rely upon certain published nonclinical or clinical studies conducted for an approved product or the FDA’s conclusions from prior review of such studies. The FDA may require companies to perform additional studies or measurements to support any changes from the approved product. The FDA may then approve the new product for all or some of the labeled indications for which the reference product has been approved, as well as for any new indication supported by the NDA. While references to nonclinical and clinical data not generated by the applicant or for which the applicant does not have a right of reference are allowed, all development, process, stability, qualification and validation data related to the manufacturing and quality of the new product must be included in an NDA submitted under Section 505(b)(2).
To the extent that the Section 505(b)(2) applicant is relying on the FDA’s conclusions regarding studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book publication. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The Section 505(b)(2) application also will not be approved until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the reference product has expired. Thus, the Section 505(b)(2) applicant may invest a significant amount of time and expense in the development of its products only to be subject to significant delay and patent litigation before its products may be commercialized.
In addition to regulating and auditing human clinical trials, the FDA regulates and inspects equipment, facilities, laboratories and processes used in the manufacturing and testing of such products prior to providing approval to market a product. If, after receiving FDA approval, we make a material change in manufacturing equipment, location or process, additional regulatory review and approval may be required. We also must adhere to cGMP regulations and product-specific regulations enforced by the FDA through its facilities inspection program. The FDA also conducts regular, periodic visits to re-inspect our equipment, facilities, laboratories and processes following the initial approval. If, as a result of these inspections, the FDA determines that our equipment, facilities, laboratories or processes do not comply with applicable FDA regulations and conditions of product approval, the FDA may seek civil, criminal or administrative sanctions and/or remedies against us, including the suspension of our manufacturing operations.
We have currently received no approvals to market our products from the FDA or other foreign regulators.
We are also subject to various federal, state and international laws pertaining to healthcare “fraud and abuse,” including anti-kickback laws and false claims laws. The federal anti-kickback law, which governs federal healthcare programs (e.g., Medicare, Medicaid), makes it illegal to solicit, offer, receive or pay any remuneration in exchange for, or to induce, the referral of business, including the purchase or prescription of a particular drug. Many states have similar laws that are not restricted to federal healthcare programs. Federal and state false claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented for payment to third party payers (including Medicare and Medicaid), claims for reimbursement, including claims for the sale of drugs or services, that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. If the government or a whistleblower were to allege that we violated these laws there could be a material adverse effect on us, including our stock price. Even an unsuccessful challenge could cause adverse publicity and be costly to respond to, which could have a materially adverse effect on our business, results of operations and financial condition. A finding of liability under these laws can have significant adverse financial implications for us and can result in payment of large penalties and possible exclusion from federal healthcare programs. We will consult counsel concerning the potential application of these and other laws to our business and our sales, marketing and other activities and will make good faith efforts to comply with them. However, given their broad reach and the increasing attention given by law enforcement authorities, we cannot assure you that some of our activities will not be challenged or deemed to violate some of these laws.
67
European Union
Regulation and Marketing Authorization in the European Union
The process governing approval of medicinal products in the European Union follows essentially the same lines as in the United States and, likewise, generally involves satisfactorily completing each of the following:
| ● | preclinical laboratory tests, animal studies and formulation studies all performed in accordance with the applicable E.U. Good Laboratory Practice regulations; | |
| ● | submission to the relevant national authorities of a clinical trial application, or CTA, which must be approved before human clinical trials may begin; | |
| ● | performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product for each proposed indication; | |
| ● | submission to the relevant competent authorities of a marketing authorization application, or MAA, which includes the data supporting safety and efficacy as well as detailed information on the manufacture and composition of the product in clinical development and proposed labelling; |
| ● | satisfactory completion of an inspection by the relevant national authorities of the manufacturing facility or facilities, including those of third parties, at which the product is produced to assess compliance with strictly enforced current cGMP; | |
| ● | potential audits of the non-clinical and clinical trial sites that generated the data in support of the MAA; and |
| ● | review and approval by the relevant competent authority of the MAA before any commercial marketing, sale or shipment of the product. |
Preclinical Studies
Preclinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animal studies, in order to assess the potential safety and efficacy of the product. The conduct of the preclinical tests and formulation of the compounds for testing must comply with the relevant E.U. and/or Member States’ regulations and requirements. The results of the preclinical tests, together with relevant manufacturing information and analytical data, are submitted as part of the CTA.
Clinical Trial Approval
Requirements for the conduct of clinical trials in the European Union including Good Clinical Practice, or GCP, are implemented in the Clinical Trials Directive 2001/20/EC and the GCP Directive 2005/28/EC. Pursuant to Directive 2001/20/EC and Directive 2005/28/EC, as amended, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the member states. Under this system, approval must be obtained from the competent national authority of an E.U. member state in which a study is planned to be conducted or in multiple member states if the clinical trial is to be conducted in a number of member states. To this end, a CTA is submitted, which must be supported by an investigational medicinal product dossier, or IMPD, and further supporting information prescribed by Directive 2001/20/EC and Directive 2005/28/EC and other applicable guidance documents. Furthermore, a clinical trial may only be started after a competent ethics committee has issued a favorable opinion on the clinical trial application in that country.
68
In April 2014, a new Clinical Trials Regulation, (EU) No 536/2014 was adopted which will replace the current Clinical Trials Directive 2001/20/EC. To ensure that the rules for clinical trials are identical throughout the European Union, the new E.U. clinical trials legislation was passed as a “regulation” that is directly applicable in all E.U. member states. All clinical trials performed in the European Union are required to be conducted in accordance with the Clinical Trials Directive 2001/20/EC until the new Clinical Trials Regulation (EU) No 536/2014 becomes applicable, which is connected to the functioning of the new E.U. Clinical Trials Database and has therefore been postponed several times. It is currently expected to be in late 2019 or 2020.
The new Regulation (EU) No 536/2014 aims to harmonize, simplify and streamline the approval of clinical trials in the European Union. The main characteristics of the Regulation include:
| ● | A streamlined application procedure via a single entry point, the E.U. portal; | |
| ● | A single set of documents to be prepared and submitted for the application as well as simplified reporting procedures that will spare sponsors from submitting broadly identical information separately to various bodies and different member states; | |
| ● | A harmonized procedure for the assessment of applications for clinical trials, which is divided in two parts. Part I is assessed jointly by all member states concerned. Part II is assessed separately by each member state concerned; | |
| ● | Strictly defined deadlines for the assessment of clinical trial application; and | |
| ● | The involvement of the ethics committees in the assessment procedure in accordance with the national law of the member state concerned but within the overall timelines defined by the Regulation (EU) No 536/2014. |
Marketing Authorization
Authorization to market a product in the member states of the European Union proceeds under one of four procedures: a centralized authorization procedure, a mutual recognition procedure, a decentralized procedure or a national procedure.
Centralized Authorization Procedure
The centralized procedure enables applicants to obtain a marketing authorization that is valid in all E.U. member states based on a single application. Certain medicinal products, including products developed by means of biotechnological processes, must undergo the centralized authorization procedure for marketing authorization which, if granted by the European Commission, is automatically valid in all 28 E.U. member states. The EMA and the European Commission administer this centralized authorization procedure pursuant to Regulation (EC) No 726/2004.
Pursuant to Regulation (EC) No 726/2004, this procedure is inter alia mandatory for:
| ● | medicinal products developed by means of one of the following biotechnological processes: |
| ● | recombinant DNA technology; | |
| ● | controlled expression of genes coding for biologically active proteins in prokaryotes and eukaryotes including transformed mammalian cells; and | |
| ● | hybridoma and monoclonal antibody methods; |
| ● | advanced therapy medicinal products as defined in Article 2 of Regulation (EC) No. 1394/2007 on advanced therapy medicinal products; |
69
| ● | medicinal products for human use containing a new active substance that, on the date of effectiveness of this regulation, was not authorized in the European Union, and for which the therapeutic indication is the treatment of any of the following diseases: |
| ● | acquired immune deficiency syndrome; | |
| ● | cancer; | |
| ● | neurodegenerative disorder; | |
| ● | diabetes; | |
| ● | auto-immune diseases and other immune dysfunctions; and | |
| ● | viral diseases; and |
| ● | medicinal products that are designated as orphan medicinal products pursuant to Regulation (EC) No 141/2000. |
The centralized authorization procedure is optional for other medicinal products if they contain a new active substance or if the applicant shows that the medicinal product concerned constitutes a significant therapeutic, scientific or technical innovation or that the granting of authorization is in the interest of patients in the European Union.
Administrative Procedure
Under the centralized authorization procedure, the EMA’s Committee for Human Medicinal Products, or CHMP, serves as the scientific committee that renders opinions about the safety, efficacy and quality of medicinal products for human use on behalf of the EMA. The CHMP is composed of experts nominated by each member state’s national authority for medicinal products, with expert appointed to act as Rapporteur for the co-ordination of the evaluation with the possible assistance of a further member of the Committee acting as a Co-Rapporteur. After approval, the Rapporteur(s) continue to monitor the product throughout its life cycle. The CHMP has 210 days to adopt an opinion as to whether a marketing authorization should be granted. The process usually takes longer in case additional information is requested, which triggers clock-stops in the procedural timelines. The process is complex and involves extensive consultation with the regulatory authorities of member states and a number of experts. When an application is submitted for a marketing authorization in respect of a drug that is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation, the applicant may pursuant to Article 14(9) Regulation (EC) No 726/2004 request an accelerated assessment procedure. If the CHMP accepts such request, the time-limit of 210 days will be reduced to 150 days but it is possible that the CHMP can revert to the standard time-limit for the centralized procedure if it considers that it is no longer appropriate to conduct an accelerated assessment. Once the procedure is completed, a European Public Assessment Report, or EPAR, is produced. If the opinion is negative, information is given as to the grounds on which this conclusion was reached. After the adoption of the CHMP opinion, a decision on the MAA must be adopted by the European Commission, after consulting the E.U. member states.
Conditional Approval
In specific circumstances, E.U. legislation (Article 14(7) Regulation (EC) No 726/2004 and Regulation (EC) No 507/2006 on Conditional Marketing Authorisations for Medicinal Products for Human Use) enables applicants to obtain a conditional marketing authorization prior to obtaining the comprehensive clinical data required for an application for a full marketing authorization. Such conditional approvals may be granted for product candidates (including medicines designated as orphan medicinal products) if (1) the risk-benefit balance of the product candidate is positive, (2) it is likely that the applicant will be in a position to provide the required comprehensive clinical trial data, (3) the product fulfills unmet medical needs and (4) the benefit to public health of the immediate availability on the market of the medicinal product concerned outweighs the risk inherent in the fact that additional data are still required. A conditional marketing authorization may contain specific obligations to be fulfilled by the marketing authorization holder, including obligations with respect to the completion of ongoing or new studies, and with respect to the collection of pharmacovigilance data. Conditional marketing authorizations are valid for one year, and may be renewed annually, if the risk-benefit balance remains positive, and after an assessment of the need for additional or modified conditions and/or specific obligations. The timelines for the centralized procedure described above also apply with respect to the review by the CHMP of applications for a conditional marketing authorization.
70
Marketing Authorization under Exceptional Circumstances
Under Article 14(8) Regulation (EC) No 726/2004, products for which the applicant can demonstrate that comprehensive data (in line with the requirements laid down in Annex I of Directive 2001/83/EC, as amended) cannot be provided (due to specific reasons foreseen in the legislation) might be eligible for marketing authorization under exceptional circumstances. This type of authorization is reviewed annually to reassess the risk-benefit balance. The fulfillment of any specific procedures/obligations imposed as part of the marketing authorization under exceptional circumstances is aimed at the provision of information on the safe and effective use of the product and will normally not lead to the completion of a full dossier/approval.
Market Authorizations Granted by Authorities of E.U. Member States
In general, if the centralized procedure is not followed, there are three alternative procedures as prescribed in Directive 2001/83/EC:
| ● | The decentralized procedure allows applicants to file identical applications to several E.U. member states and receive simultaneous national approvals based on the recognition by E.U. member states of an assessment by a reference member state; | |
| ● | The national procedure is only available for products intended to be authorized in a single E.U. member state; and | |
| ● | A mutual recognition procedure similar to the decentralized procedure is available when a marketing authorization has already been obtained in at least one E.U. member state. |
A marketing authorization may be granted only to an applicant established in the European Union.
Pediatric Studies
Prior to obtaining a marketing authorization in the European Union, applicants have to demonstrate compliance with all measures included in an EMA-approved Paediatric Investigation Plan, or PIP, covering all subsets of the paediatric population, unless the EMA has granted a product-specific waiver, a class waiver, or a deferral for one or more of the measures included in the PIP. The respective requirements for all marketing authorization procedures are set forth in Regulation (EC) No 1901/2006, which is referred to as the Pediatric Regulation. This requirement also applies when a company wants to add a new indication, pharmaceutical form or route of administration for a medicine that is already authorized. The Pediatric Committee of the EMA, or PDCO, may grant deferrals for some medicines, allowing a company to delay development of the medicine in children until there is enough information to demonstrate its effectiveness and safety in adults. The PDCO may also grant waivers when development of a medicine in children is not needed or is not appropriate, such as for diseases that only affect the elderly population.
Before a marketing authorization application can be filed, or an existing marketing authorization can be amended, the EMA determines that companies actually comply with the agreed studies and measures listed in each relevant PIP.
71
Periods of Authorization and Renewals
A marketing authorization is valid for five years in principle and the marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the competent authority of the authorizing member state. To this end, the marketing authorization holder must provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases to be valid. Once renewed, the marketing authorization is valid for an unlimited period, unless the European Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal. Any authorization which is not followed by the actual placing of the drug on the E.U. market (in case of centralized procedure) or on the market of the authorizing member state within three years after authorization ceases to be valid (the so-called sunset clause).
Orphan Drug Designation and Exclusivity
Pursuant to Regulation (EC) No 141/2000 and Regulation (EC) No. 847/2000, the European Commission can grant such orphan medicinal product designation to products for which the sponsor can establish that it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in 10,000 people in the European Union, or a life threatening, seriously debilitating or serious and chronic condition in the European Union and with regards to that without incentives it is unlikely that sales of the drug in the European Union would generate a sufficient return to justify the necessary investment. In addition, the sponsor must establish that there is no other satisfactory method approved in the European Union of diagnosing, preventing or treating the condition, or if such a method exists, the proposed orphan drug will be of significant benefit to patients.
Orphan drug designation is not a marketing authorization. It is a designation that provides a number of benefits, including fee reductions, regulatory assistance, and the possibility to apply for a centralized E.U. marketing authorization, as well as ten years of market exclusivity following a marketing authorization. During this market exclusivity period, neither the EMA, the European Commission nor the member states can accept an application or grant a marketing authorization for a “similar medicinal product.” A “similar medicinal product” is defined as a medicinal product containing a similar active substance or substances as those contained in an authorized orphan medicinal product and that is intended for the same therapeutic indication. The market exclusivity period for the authorized therapeutic indication may be reduced to six years if, at the end of the fifth year, it is established that the orphan designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity. In addition, a competing similar medicinal product may in limited circumstances be authorized prior to the expiration of the market exclusivity period, including if the marketing authorization holder is unable to supply sufficient quantities of the product or if the competing product is shown to be safer, more effective or otherwise clinically superior to the already approved orphan drug. Furthermore, a product can lose orphan designation, and the related benefits, prior to us obtaining a marketing authorization if it is demonstrated that the orphan designation criteria are no longer met.
Regulatory Data Protection
E.U. legislation also provides for a system of regulatory data and market exclusivity. According to Article 14(11) of Regulation (EC) No 726/2004, as amended, and Article 10(1) of Directive 2001/83/EC, as amended, upon receiving marketing authorization, new chemical entities approved on the basis of a complete independent data package benefit from eight years of data exclusivity and an additional two years of market exclusivity. Data exclusivity prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a generic (abbreviated) application. During the additional two-year period of market exclusivity, a generic marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic medicinal product can be marketed until the expiration of the market exclusivity. The overall ten-year period will be extended to a maximum of 11 years if, during the first eight years of those ten years, the marketing authorization holder, or MAH, obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Even if a compound is considered to be a new chemical entity and the innovator is able to gain the period of data exclusivity, another company nevertheless could also market another version of the drug if such company obtained marketing authorization based on an MAA with a complete independent data package of pharmaceutical tests, preclinical tests and clinical trials. However, products designated as orphan medicinal products enjoy, upon receiving marketing authorization, a period of ten years of orphan market exclusivity—see also Orphan Drug Designation and Exclusivity. Depending upon the timing and duration of the E.U. marketing authorization process, products may be eligible for up to five years’ supplementary protection certificates, or SPCs, pursuant to Regulation (EC) No 469/2009. Such SPCs extend the rights under the basic patent for the drug (see below sub Patent Term Extension).
72
Regulatory Requirements After a Marketing Authorization has been Obtained
If we obtain authorization for a medicinal product in the European Union, we will be required to comply with a range of requirements applicable to the manufacturing, marketing, promotion and sale of medicinal products:
Pharmacovigilance and other requirements
We will, for example, have to comply with the E.U.’s stringent pharmacovigilance or safety reporting rules, pursuant to which post-authorization studies and additional monitoring obligations can be imposed. Other requirements relate, for example, to the manufacturing of products and APIs in accordance with good manufacturing practice standards. E.U. regulators may conduct inspections to verify our compliance with applicable requirements, and we will have to continue to expend time, money and effort to remain compliant. Non-compliance with E.U. requirements regarding safety monitoring or pharmacovigilance, and with requirements related to the development of products for the pediatric population, can also result in significant financial penalties in the European Union. Similarly, failure to comply with the E.U.’s requirements regarding the protection of individual personal data can also lead to significant penalties and sanctions. Individual E.U. member states may also impose various sanctions and penalties in case we do not comply with locally applicable requirements.
Manufacturing
The manufacturing of authorized drugs, for which a separate manufacturer’s license is mandatory, must be conducted in strict compliance with the EMA’s Good Manufacturing Practices, or cGMP, requirements and comparable requirements of other regulatory bodies in the European Union, which mandate the methods, facilities and controls used in manufacturing, processing and packing of drugs to assure their safety and identity. The EMA enforces its current cGMP requirements through mandatory registration of facilities and inspections of those facilities. The EMA may have a coordinating role for these inspections while the responsibility for carrying them out rests with the member states competent authority under whose responsibility the manufacturer falls. Failure to comply with these requirements could interrupt supply and result in delays, unanticipated costs and lost revenues, and could subject the applicant to potential legal or regulatory action, including but not limited to warning letters, suspension of manufacturing, seizure of product, injunctive action or possible civil and criminal penalties.
Marketing and Promotion
The marketing and promotion of authorized drugs, including industry-sponsored continuing medical education and advertising directed toward the prescribers of drugs and/or the general public, are strictly regulated in the European Union under Directive 2001/83/EC. The applicable regulations aim to ensure that information provided by holders of marketing authorizations regarding their products is truthful, balanced and accurately reflects the safety and efficacy claims authorized by the EMA or by the competent authority of the authorizing member state. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties.
Patent Term Extension
In order to compensate the patentee for delays in obtaining a marketing authorization for a patented product, a supplementary certificate, or SPC, may be granted extending the exclusivity period for that specific product by up to five years. Applications for SPCs must be made to the relevant patent office in each E.U. member state and the granted certificates are valid only in the member state of grant. An application has to be made by the patent owner within six months of the first marketing authorization being granted in the European Union (assuming the patent in question has not expired, lapsed or been revoked) or within six months of the grant of the patent (if the marketing authorization is granted first). In the context of SPCs, the term “product” means the active ingredient or combination of active ingredients for a medicinal product and the term “patent” means a patent protecting such a product or a new manufacturing process or application for it. The duration of an SPC is calculated as the difference between the patent’s filing date and the date of the first marketing authorization, minus five years, subject to a maximum term of five years.
A six month pediatric extension of an SPC may be obtained where the patentee has carried out an agreed pediatric investigation plan, the authorized product information includes information on the results of the studies and the product is authorized in all member states of the European Union.
73
Israel
Israel Ministry of the Environment — Toxin Permit
In accordance with the Israeli Dangerous Substance Law — 1993, the Ministry of the Environment may grant a permit in order to use toxic materials. Because we utilize toxic materials in the course of operation of our laboratories, we were required to apply for a permit to use these materials. Our current toxin permit will remain in effect until January 2020.
Other Licenses and Approvals
We have a business license from the municipality of Petah-Tikva for a drug development research laboratory located at our offices in Petah Tikva, Israel. In order to obtain this license, we also received approval from the Petah-Tikva Association of Towns Fire Department. The business license is valid until December 31, 2019. We also have a radioactive materials or products containing radioactive materials license, which is valid until July 25, 2019.
In 2002, we received approval from the National Council on Animal Experiments, approving us as an institution authorized to conduct experiments on animals.
Clinical Testing in Israel
In order to conduct clinical testing on humans in Israel, special authorization must first be obtained from the ethics committee and general manager of the institution in which the clinical studies are scheduled to be conducted, as required under the Guidelines for Clinical Trials in Human Subjects implemented pursuant to the Israeli Public Health Regulations (Clinical Trials in Human Subjects), as amended from time to time, and other applicable legislation. These regulations also require authorization from the Israeli Ministry of Health, except in certain circumstances, and in the case of genetic trials, special fertility trials and similar trials, an additional authorization of the overseeing institutional ethics committee. The institutional ethics committee must, among other things, evaluate the anticipated benefits that are likely to be derived from the project to determine if it justifies the risks and inconvenience to be inflicted on the human subjects, and the committee must ensure that adequate protection exists for the rights and safety of the participants as well as the accuracy of the information gathered in the course of the clinical testing. Since we intend to perform a portion of the clinical studies on certain of our product candidates in Israel, we will be required to obtain authorization from the ethics committee and general manager of each institution in which we intend to conduct our clinical trials, and in most cases, from the Israeli Ministry of Health.
Israel Ministry of Health
Israel’s Ministry of Health, which regulates medical testing, has adopted protocols that correspond, generally, to those of the FDA and the EMA, making it comparatively straightforward for studies conducted in Israel to satisfy FDA and the European Medicines Agency requirements, thereby enabling medical technologies subjected to clinical trials in Israel to reach U.S. and EU commercial markets in an expedited fashion. Many members of Israel’s medical community have earned international prestige in their chosen fields of expertise and routinely collaborate, teach and lecture at leading medical centers throughout the world. Israel also has free trade agreements with the United States and the European Union.
74
Other Countries
In addition to regulations in the United States, the EU and Israel, we are subject to a variety of other regulations governing clinical trials and commercial sales and distribution of drugs in other countries. Whether or not our products receive approval from the FDA, approval of such products must be obtained by the comparable regulatory authorities of countries other than the United States before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials and product licensing vary greatly from country to country.
The requirements that we and our collaborators must satisfy to obtain regulatory approval by government agencies in other countries prior to commercialization of our products in such countries can be rigorous, costly and uncertain. In Canada and Australia, regulatory requirements and approval processes are similar in principle to those in the United States. For example, in Canada, pharmaceutical product candidates are regulated by the Food and Drugs Act and the rules and regulations promulgated thereunder, which are enforced by Health Canada. Before commencing clinical trials in Canada, an applicant must complete preclinical studies and file a clinical trial application with Health Canada. After filing a clinical trial application, the applicant must receive different clearance authorizations to proceed with Phase 1 clinical trials, which can then lead to Phase 2 and Phase 3 clinical trials. To obtain regulatory approval to commercialize a new drug in Canada, a new drug submission, or NDS, must be filed with Health Canada. If the NDS demonstrates that the product was developed in accordance with the regulatory authorities’ rules, regulations and guidelines and demonstrates favorable safety and efficacy and receives a favorable risk/benefit analysis, Health Canada issues a notice of compliance which allows the applicant to market the product. Facilities, procedures, operations and/or testing of products are subject to periodic inspection by Health Canada and the Health Products and Food Branch Inspectorate. In addition, Health Canada conducts pre-approval and post-approval reviews and plant inspections to determine whether systems are in compliance with the good manufacturing practices in Canada, Drug Establishment Licensing requirements and other provisions of the Food and Drug Regulations.
Foreign governments also have stringent post-approval requirements including those relating to manufacture, labeling, reporting, record keeping and marketing. Failure to substantially comply with these on-going requirements could lead to government action against the product, our company and/or our representatives.
Related Matters
From time to time, legislation is drafted, introduced and passed in governmental bodies that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA, the EMA, the Israeli Ministry of Health and other applicable regulatory bodies to which we are subject. In addition, regulations and guidance are often revised or reinterpreted by the national agency in ways that may significantly affect our business and our product candidates. It is impossible to predict whether such legislative changes will be enacted, whether FDA, EMA or Israeli Ministry of Health regulations, guidance or interpretations will change, or what the impact of such changes, if any, may be. We may need to adapt our business and product candidates and products to changes that occur in the future.
C. Organizational Structure
Our corporate structure consists of Can-Fite and two wholly owned subsidiaries which are both inactive: Ultratrend Limited, incorporated in England and Wales, and Eye-Fite Limited, incorporated in Israel.
D. Property, Plants and Equipment
We are headquartered in Petah-Tikva, Israel. We lease one floor in one facility pursuant to a lease agreement with Eshkolit Nihul Nadlan LTD, an Israeli limited company. Pursuant to a verbal agreement with the lessor, the lease expires on December 31, 2019. The Petah-Tikva headquarters consists of approximately 300 square meters of space. Lease payments are approximately NIS 20,447, or $5,318, per month. If our lease is terminated, we do not foresee significant difficulty in leasing another suitable facility. The current facility houses our administrative, clinical and research operations. The research laboratory consists of approximately 150 square meters and includes a tissue culture laboratory and a molecular biology laboratory.
75
ITEM 4A. Unresolved Staff Comments
Not Applicable.
ITEM 5. Operating and Financial Review and Prospects
The information in this section should be read in conjunction with our consolidated financial statements and related notes beginning on page F-1 and the related information included elsewhere in this Annual Report on Form 20-F. Our financial statements are prepared in accordance with IFRS as issued by the International Accounting Standards Board, and reported in U.S. dollars. We maintain our accounting books and records in U.S. dollars and our functional currency is the U.S. dollar. Certain amounts presented herein may not sum due to rounding.
Overview
We are a clinical-stage biopharmaceutical company focused on developing orally bioavailable small molecule therapeutic products for the treatment of cancer, liver and inflammatory disease and sexual dysfunction. Our platform technology utilizes the Gi protein associated A3AR as a therapeutic target. A3AR is highly expressed in inflammatory and cancer cells, and not significantly expressed in normal cells, suggesting that the receptor could be a unique target for pharmacological intervention. Our pipeline of drug candidates are synthetic, highly specific agonists and allosteric modulators, or ligands or molecules that initiate molecular events when binding with target proteins, targeting the A3AR.
Our product candidates, CF101, CF102 and CF602, are being developed to treat autoimmune inflammatory indications, oncology and liver diseases as well as sexual dysfunction. CF101, also known as Piclidenoson, is in an advance stage of clinical development for the treatment of autoimmune-inflammatory diseases, including rheumatoid arthritis and psoriasis. CF102, also known as Namodenoson, is being developed for the treatment of HCC and has orphan drug designation for the treatment of HCC in the United States and Europe. Namodenoson was granted Fast Track designation by the FDA as a second line treatment to improve survival for patients with advanced HCC who have previously received Nexavar (sorafenib). Namodenoson is also being developed for the treatment of NASH, following our study which revealed compelling pre-clinical data on Namodenoson in the treatment of NASH, a disease for which no FDA approved therapies currently exist. CF602 is our second generation allosteric drug candidate for the treatment of sexual dysfunction, which has shown efficacy in the treatment of erectile dysfunction in preclinical studies and we are investigating additional compounds, targeting A3AR, for the treatment of sexual dysfunction. Preclinical studies revealed that our drug candidates have potential to treat additional inflammatory diseases, such as Crohn’s disease, oncological diseases, viral diseases, such as the JC virus, and obesity.
We believe our pipeline of drug candidates represent a significant market opportunity. For instance, according to Visiongain, the world rheumatoid arthritis market size is predicted to generate revenues of $34.6 billion in 2020 and the psoriasis drug market is forecasted to be worth $11.4 billion by 2020. According to DelveInsight, the HCC drug market in the G8 countries (U.S., Germany, France, Italy, Spain, UK, Japan and China) is expected to reach $3.8 billion by 2027.
We have in-licensed an allosteric modulator of the A3AR, CF602 from Leiden University. In addition, we have out-licensed the following:
| ● | Piclidenoson for the treatment of (i) rheumatoid arthritis to Kwang Dong Pharmaceutical Co. Ltd. for Korea, (ii) psoriasis and rheumatoid arthritis to Cipher Pharmaceuticals for Canada, (iii) rheumatoid arthritis and psoriasis to Gebro Holding, for Spain, Switzerland and Austria, and (iv) rheumatoid arthritis and psoriasis to CMS Medical for China (including Hong Kong, Macao and Taiwan); and |
| ● | Namodenoson for the treatment of (i) liver cancer and NASH to Chong Kun Dang Pharmaceuticals for South Korea, and (ii) advanced liver cancer and NAFLD/NASH to CMS Medical for China (including Hong Kong, Macao and Taiwan). |
76
We are currently: (i) conducting a Phase III trial for Piclidenoson in the treatment of rheumatoid arthritis, (ii) conducting a Phase III trial for Piclidenoson in the treatment of psoriasis, (iii) completing the analysis of the results of our Phase II advanced liver cancer study having recently released top-line results, (iv) conducting a Phase II trial of Namodenoson in the treatment of NASH top-line results expected in the second half of 2019, and (v) investigating additional compounds, targeting the A3 adenosine receptor, for the treatment of sexual dysfunction and have therefore postponed a planned Investigational New Drug (IND) submission for this indication.
Since inception, we have incurred significant losses in connection with our research and development. As of December 31, 2018, we had an accumulated deficit of approximately $100.6 million. Although we have recognized revenues in connection with our existing out-licensing agreements with KD, Cipher, CKD, Gebro and CMS and our historic out-licensing agreement with SKK, we expect to generate losses in connection with the research and development activities relating to our pipeline of drug candidates. Such research and development activities are budgeted to expand over time and will require further resources if we are to be successful. As a result, we expect to incur operating losses, which may be substantial over the next several years, and we will need to obtain additional funds to further develop our research and development programs.
We have funded our operations primarily through the sale of equity securities (both in private placements and in public offerings) and payments received under our existing out-licensing agreements with KD, Cipher, CKD, Gebro and CMS and our historic out-licensing agreement with SKK. We expect to continue to fund our operations over the next several years through our existing cash resources, potential future milestone payments that we expect to receive from our licensees, interest earned on our investments, if any, and additional capital to be raised through public or private equity offerings or debt financings. As of December 31, 2018, we had approximately $3.61 million of cash and cash equivalents.
Revenues
Our revenues to date have been generated primarily from payments under our existing out-licensing agreements with KD, Cipher, CKD, Gebro and CMS and our historic out-licensing agreement with SKK.
Under the Kwang Dong License Agreement, we are entitled to up-front and milestone payments of up to $1.5 million. In accordance with the Kwang Dong License Agreement, we received an up-front payment of $0.3 million and a payment of $0.048 million as consideration for KD’s purchase of our ordinary shares in 2009 and a milestone payment of $0.2 million in 2010. Under the terms of the Kwang Dong License Agreement, in addition to the payments mentioned above, we are entitled to certain additional payments based on the sale of raw materials, subject to the terms and conditions of the respective agreements. To date, we have received a total of $500,000 from Kwang Dong in an upfront payment. See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”.
Under the Distribution and Supply Agreement with Cipher we received CAD 1.65 million upon execution of the agreement and are entitled to milestone payments upon receipt of regulatory approval by Health Canada for Piclidenoson and the first delivery of commercial launch quantities as follows (i) CAD 1 million upon the first approved indication for either psoriasis or rheumatoid arthritis, and (ii) CAD 1 million upon the second approved indication for either psoriasis or rheumatoid arthritis. In addition, following regulatory approval, we shall be entitled to a royalty of 16.5% of net sales of Piclidenoson in Canada and reimbursement for the cost of manufacturing Piclidenoson. We are also entitled to a royalty payment for any authorized generic of Piclidenoson that Cipher distributes in Canada. To date, we have received a total of $1.3 million from Cipher in an upfront payment. See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”.
The Distribution Agreement with CKD provides for up to $3,000,000 in upfront and milestone payments payable with respect to the liver cancer indication and up to $3,000,000 with respect to the NASH indication. In addition, we are entitled to a transfer price of the higher of the manufacturing cost plus 10% or 23% of net sales of Namodenoson following commercial launch in South Korea. To date, we have received a total of $1,000,000 from CKD, $500,000 in upfront payments and a further $500,000 for a milestone payment received in the third quarter of 2017 upon receipt by CKD of a positive result from the preliminary review by the MFDS on obtaining orphan drug designation in South Korea. See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”.
77
In January 2018, we entered into a Distribution and Supply Agreement with Gebro. The Distribution and Supply Agreement with Gebro provides that we are entitled to €1,500,000 upon execution of the agreement plus milestone payments upon achieving certain clinical, launch and sales milestones, as follows: (i) €300,000 upon initiation of the ACRobat Phase III clinical trial for the treatment of rheumatoid arthritis and €300,000 upon the initiation of the COMFORT Phase III clinical trial for the treatment of psoriasis, (ii) between €750,000 and €1,600,000 following first delivery of commercial launch quantities of Piclidenson for either the treatment of rheumatoid arthritis or psoriasis, and (iii) between €300,000 and up to €4,025,000 upon meeting certain net sales. In addition, following regulatory approval, we shall be entitled to double digit percentage royalties on net sales of Piclidenoson in the territories and payment for the manufacturing Piclidenoson. To date, we have received a total of €2,100,000 from Gebro in upfront and milestone payments. See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”.
In August 2018, we entered into a License, Collaboration and Distribution Agreement with CMS Medical. Under the License, Collaboration and Distribution Agreement, we are entitled to $2,000,000 upon execution of the agreement plus milestone payments of up to $14,000,000 upon achieving certain regulatory milestones and payments of up to $58,500,000 upon achieving certain sales milestones. In addition, following regulatory approval, we shall be entitled to double-digit percentage royalties on net sales of Piclidenoson and Namodenoson in the licensed territories. To date, we have received a total of $2,000,000 from CMS Medical in upfront and milestone payments. See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”.
Under the terminated SKK license agreement we received an aggregate of approximately $8.5 million from SKK. See “tem 4. Information on the Company—B. Business—Out-Licensing and Distribution Agreements”.
Certain payments we have received from SKK and KD have been subject to a 10% and 5% withholding tax in Japan and Korea, respectively, and certain payments we may receive in the future, if at all, may also be subject to the same withholding tax in Korea. Receipt of any milestone payment under our out-licensing agreements depends on many factors, some of which are beyond our control. We cannot assure you that we will receive any of these future payments. We expect our revenues for the next several years, if any, to be derived primarily from payments under our current out-license agreements and our public capital raising activities, as well as additional collaborations that we may enter into in the future with respect to our drug candidates.
Research and Development
Our research and development expenses consist primarily of salaries and related personnel expenses, fees paid to external service providers, up-front and milestone payments under our license agreements, patent-related legal fees, costs of preclinical studies and clinical trials, drug and laboratory supplies and costs for facilities and equipment. We charge all research and development expenses to operations as they are incurred. We expect our research and development expense to remain our primary expense in the near future as we continue to develop our products. Increases or decreases in research and development expenditures are attributable to the number and/or duration of the pre-clinical and clinical studies that we conduct.
The following table identifies our current major research and development projects:
| Project | Status | Expected or Recent Near Term Milestone | ||
| Piclidenoson | ACRobat Phase III study in rheumatoid arthritis | Enrolling patients to the study | ||
| COMFORT Phase III study in psoriasis | Enrolling patients to the study | |||
| Namodenoson | Phase II in HCC | Top-line results announced in March 2019 | ||
| Phase II study in NASH | Top-line results expected in second half of 2019 |
78
We record certain costs for each development project on a “direct cost” basis, as they are recorded to the project for which such costs are incurred. Such costs include, but are not limited to, CRO expenses, drug production for pre-clinical and clinical studies and other pre-clinical and clinical expenses. However, certain other costs, including but not limited to, salary expenses (including salaries for research and development personnel), facilities, depreciation, share-based compensation and other overhead costs are recorded on an “indirect cost” basis, i.e., they are shared among all of our projects and are not recorded to the project for which such costs are incurred. We do not allocate direct salaries to projects due to the fact that our project managers are generally involved in several projects at different stages of development, and the related salary expense is not significant to the overall cost of the applicable projects. In addition, indirect labor costs relating to our support of the research and development process, such as manufacturing, controls, pre-clinical analysis, laboratory testing and initial drug sample production, as well as rent and other administrative overhead costs, are shared by many different projects and have never been considered by management to be of significance in its decision-making process with respect to any specific project. Accordingly, such costs have not been specifically allocated to individual projects.
Set forth below is a summary of the gross direct costs allocated to our main projects on an individual basis, as well as the gross direct costs allocated to our less significant projects on an aggregate basis, for the years ended December 31, 2016, 2017 and 2018; and on an aggregate basis since project inception:
| (USD in thousands) | Total Costs | |||||||||||||||
| Year Ended December 31, | Since Project | |||||||||||||||
| 2016 | 2017 | 2018 | Inception | |||||||||||||
| Piclidenoson | 1,946 | 1,894 | 2,987 | 26,362 | ||||||||||||
| Namodenoson | 1,907 | 1,827 | 1,103 | 8,558 | ||||||||||||
| CF602 | 1,126 | 15 | 276 | 1,683 | ||||||||||||
| Other projects | - | - | - | 1,729 | ||||||||||||
| Total gross direct project costs (1) | 4,979 | 3,736 | 4,366 | 38,332 | ||||||||||||
| (1) | Does not include indirect project costs and overhead, such as payroll and related expenses (including stock-based compensation), facilities, depreciation and impairment of intellectual property, which are included in total research and development expenses in our financial statements. |
From our inception through December 31, 2018, we have incurred research and development expenses of approximately $99.8 million. We expect that a large percentage of our research and development expense in the future will be incurred in support of our current and future preclinical and clinical development projects. Due to the inherently unpredictable nature of preclinical and clinical development processes and given the early stage of our preclinical product development projects, we are unable to estimate with any certainty the costs we will incur in the continued development of the product candidates in our pipeline for potential commercialization. Clinical development timelines, the probability of success and development costs can differ materially from expectations. We expect to continue to test our product candidates in preclinical studies for toxicology, safety and efficacy, and to conduct additional clinical trials for each product candidate. If we are not able to enter into an out-licensing arrangement with respect to any product candidate prior to the commencement of later stage clinical trials, we may fund the trials for the product candidates ourselves.
While we are currently focused on advancing each of our product development projects, our future research and development expenses will depend on the clinical success of each product candidate, as well as ongoing assessments of each product candidate’s commercial potential. In addition, we cannot forecast with any degree of certainty which product candidates may be subject to future out-licensing arrangements, when such out-licensing arrangements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements.
As we obtain results from clinical trials, we may elect to discontinue or delay clinical trials for certain product candidates or projects in order to focus our resources on more promising product candidates or projects. Completion of clinical trials by us or our licensees may take several years or more, but the length of time generally varies according to the type, complexity, novelty and intended use of a product candidate.
79
The cost of clinical trials may vary significantly over the life of a project as a result of differences arising during clinical development, including, among others:
| ● | the number of sites included in the clinical trials; |
| ● | the length of time required to enroll suitable patients; |
| ● | the number of patients that participate in the clinical trials; |
| ● | the duration of patient follow-up; |
| ● | the development stage of the product candidate; and |
| ● | the efficacy and safety profile of the product candidate. |
We expect our research and development expenses to increase in the future from current levels as we continue the advancement of our clinical trials and preclinical product development and to the extent we in-license new product candidates. The lengthy process of completing clinical trials and seeking regulatory approval for our product candidates requires expenditure of substantial resources. Any failure or delay in completing clinical trials, or in obtaining regulatory approvals, could cause a delay in generating product revenue and cause our research and development expenses to increase and, in turn, have a material adverse effect on our operations. Because of the factors set forth above, we are not able to estimate with any certainty when we would recognize any net cash inflows from our projects.
General and Administrative Expenses
General and administrative expenses consist primarily of compensation for employees in executive and operational functions, including accounting, finance, legal, business development, investor relations, information technology and human resources. Other significant general and administration costs include facilities costs, professional fees for outside accounting and legal services, travel costs, insurance premiums and depreciation.
Financial Expense and Income
Financial expense and income consists of interest earned on our cash and cash equivalents; bank fees and other transactional costs; expense or income resulting from fluctuations of the NIS and other currencies, in which a portion of our assets and liabilities are denominated, against the U.S. dollar (our functional currency).
Critical Accounting Policies and Estimates
Our accounting policies and their effect on our financial condition and results of operations are more fully described in our audited consolidated financial statements included elsewhere in this Annual Report on Form 20-F. The preparation of financial statements in conformity with IFRS as issued by the IASB requires management to make estimates and assumptions that in certain circumstances affect the reported amounts of assets and liabilities, revenues and expenses and disclosure of contingent assets and liabilities. These estimates are prepared using our best judgment, after considering past and current events and economic conditions. While management believes the factors evaluated provide a meaningful basis for establishing and applying sound accounting policies, management cannot guarantee that the estimates will always be consistent with actual results. In addition, certain information relied upon by us in preparing such estimates includes internally generated financial and operating information, external market information, when available, and when necessary, information obtained from consultations with third party experts. Actual results could differ from these estimates and could have a material adverse effect on our reported results.
80
We believe that the accounting policies discussed below are critical to our financial results and to the understanding of our past and future performance, as these policies relate to the more significant areas involving management’s estimates and assumptions. We consider an accounting estimate to be critical if: (1) it requires us to make assumptions because information was not available at the time or it included matters that were highly uncertain at the time we were making our estimate; and (2) changes in the estimate could have a material impact on our financial condition or results of operations.
Functional and Presentation Currency
From our inception through January 1, 2018, our functional and presentation currency was the NIS. Management conducted a review of our functional currency and decided to change our functional and presentation currency to the USD from the NIS effective January 1, 2018. These changes were based on an assessment by our management that the USD is the primary currency of the economic environment in which we operate.
In determining the appropriate functional currency to be used, we followed the guidance in International Accounting Standard 21 - The Effects of Changes in Foreign Exchange Rates, or IAS 21, which states that factors relating to sales, costs and expenses, financing activities and cash flows, as well as other potential factors, should be considered. In this regard, we are incurring and expect to continue to incur a majority of our expenses in USD as a result of our expanded clinical trials including Phase III trials. These changes, as well as the fact that the majority of our available funds are in U.S. dollars, our principal source of financing is the U.S. capital market, and all of our budgeting is conducted solely in U.S. dollars, led to the decision to make the change in functional currency as of January 1, 2018, as indicated above.
At the date of change of functional currency, we also changed the presentation currency of our financial statements. This change was retrospectively implemented. In accordance with IAS 21, since our presentation currency was different than our functional currency our results and financial position were translated using the following principles: (i) all assets and liabilities were translated using the current exchange rates, (ii) equity accounts were translated using the historical rates, and (iii) income and expenses for each statement of comprehensive income or separate income statement presented were translated at exchange rates at the dates of the transactions.
Principles of Consolidation
Our financial statements reflect the consolidation of the financial statements of companies that we control based on legal control or effective control. We fully consolidate into our financial statements the results of operations of companies that we control. Legal control exists when we have the power, directly or indirectly, to govern the financial and operating policies of an entity. The effect of potential voting rights that are exercisable at the balance sheet date are considered when assessing whether we have legal control. In addition, we consolidate on the basis of effective control even if we do not have voting control. The determination that effective control exists involves significant judgment.
In evaluating the effective control on our investees we consider the following criteria to determine if effective control exists:
| ● | whether we hold a significant voting interest (but less than half the voting rights); | |
| ● | whether there is a wide diversity of public holdings of the remaining shares conferring voting rights; | |
| ● | whether in the past we had the majority of the voting power participating in the general meetings of shareholders and, therefore, have in fact had the right to nominate the majority of the board members; | |
| ● | the absence of a single entity that holds a significant portion of the investee’s shares; | |
| ● | our ability to establish policies and guide operations by appointing the remainder of the investee’s senior management; and | |
| ● | whether the minority shareholders have participation rights or other preferential rights, excluding traditional shareholder protective rights. | |
81
Entities we control are fully consolidated in our financial statements. All significant intercompany balances and transactions are eliminated in consolidation. Non-controlling interests of subsidiaries represent the non-controlling shareholders’ proportionate interest in the comprehensive income (loss) of the subsidiaries and fair value of the net assets or the net identifiable assets upon the acquisition of the subsidiaries.
Revenue Recognition
We generate income from distribution agreements. See “Item 4. Information on the Company—B. Business—Out-Licensing and Distribution Agreements”. Such income comprises of upfront license fees, milestone payments and potential royalty payments.
We identified four components in the agreements: (i) performing the research and development services through regulatory approval; (ii) exclusive license to distribute; (iii) participation in joint steering committee; and, (iv) royalties resulting from future sales of the product.
We recognize revenue in accordance with IFRS 15, “Revenue” pursuant to which each required deliverable is evaluated to determine whether it qualifies as a separate unit of accounting based on whether the deliverable has “stand-alone value” to the customer. The arrangement’s consideration that is fixed or determinable is then allocated to each separate unit of accounting based on the relative selling price of each deliverable which is based on the Estimated Selling Price.
Components (i) – (iii) were analyzed as one unit of accounting. Consequently, revenue from these components is recorded based on the term of the research and development services (which is the last deliverable in the arrangement). We estimate these services will spread over a period of 24 quarters.
Revenues from milestone payments:
Contingent payments related to milestones will be recognized immediately upon satisfaction of the milestone and contingent payments related to royalties will be recognized in the period that the related sales have occurred.
Revenues from royalties:
Revenues from royalties will be recognized as they accrue in accordance with the terms of the relevant agreement.
Share-based Compensation
We account for share-based compensation arrangements in accordance with the provisions of IFRS 2. IFRS 2 requires companies to recognize share-based compensation expense for awards of equity instruments based on the grant-date fair value of those awards. The cost is recognized as compensation expense over the vesting period, based upon the grant-date fair value of the equity or liability instruments issued. We selected the binomial option pricing model as the most appropriate method for determining the estimated fair value of our share-based awards without market conditions. The determination of the grant date fair value of options using an option pricing model is affected by estimates and assumptions regarding a number of complex and subjective variables. These variables include the expected volatility of our share price over the expected term of the options, share option exercise and forfeiture rate, risk-free interest rates, expected dividends and the price of our ordinary shares on the TASE. As our ordinary shares are publicly traded on the TASE, we do not need to estimate the fair value of our ordinary shares. Rather, we use the actual closing market price of our ordinary shares on the date of grant, as reported by the TASE although in the future may use the closing market price of our ADSs on the date of grant, as reported by the NYSE American.
If any of the assumptions used in the binomial option pricing model change significantly, share-based compensation for future awards may differ materially compared with the awards previously granted.
82
As for other service providers, the cost of the transactions is measured at the fair value of the goods or services received as consideration for equity instruments. In cases where the fair value of the goods or services received as consideration of equity instruments cannot be measured, they are measured by reference to the fair value of the equity instruments granted.
The cost of equity-settled transactions is recognized in profit or loss, together with a corresponding increase in equity, during the period which the service are to be satisfied, ending on the date on which the relevant employees or other service providers become fully entitled to the award.
If we modify the conditions on which equity-instruments are granted, an additional expense is recognized for any modification that increases the total fair value of the share-based payment arrangement or is otherwise beneficial to the employee or other service provider at the modification date.
Liability Related to Certain Warrants
The fair value of the liability for warrants exercisable into shares issued to investors in connection with our financings to date was calculated using the Black-Scholes-Merton option-pricing model. We accounted for these warrants as liabilities due to the dollar exercise price terms and in accordance with IAS 39, measured at fair value each reporting period until they will be exercised or expired, with changes in the fair values being recognized in our statement of comprehensive loss as financial income or expense.
Fair value for each reporting period was calculated based on the following assumptions:
| 1. | Risk-free interest rate - based on yield rate of non-index linked U.S. Federal Reserve treasury bonds. | |
| 2. | Expected volatility - was calculated based on our actual historical stock price movements over a term that is equivalent to the expected term of the option. | |
| 3. | Expected life - the expected life was based on the expiration date of the warrants. | |
| 4. | Expected dividend yield - was based on the fact that we have not paid dividends to our shareholders in the past and do not expect to pay dividends to our shareholders in the future. |
Our net loss for the year ended December 31, 2018 and 2017 included finance income in the amount of $0 and $564,000, respectively, in connection with the above-mentioned warrants.
Recently Issued Accounting Pronouncements
| IFRS 16 - Leases: |
In January 2016, the IASB issued IFRS 16, “Leases” (“IFRS 16”). According to IFRS 16, a lease is a contract, or part of a contract, that conveys the right to use an asset for a period of time in exchange for consideration.
The effects of the adoption of the new standard are as follows:
| ● | According to IFRS 16, lessees are required to recognize all leases in the statement of financial position (excluding certain exceptions, see below). Lessees will recognize a liability for lease payments with a corresponding right-of-use asset, similar to the accounting treatment for finance leases under the existing standard, IAS 17, “Leases”. Lessees will also recognize interest expense and depreciation expense separately. |
| ● | Variable lease payments that are not dependent on changes in the Consumer Price Index (“CPI”) or interest rates, but are based on performance or use are recognized as an expense by the lessees as incurred and recognized as income by the lessors as earned. |
83
| ● | In the event of change in variable lease payments that are CPI-linked, lessees are required to remeasure the lease liability and record the effect of the remeasurement as an adjustment to the carrying amount of the right-of-use asset. |
| ● | The accounting treatment by lessors remains substantially unchanged from the existing standard, namely classification of a lease as a finance lease or an operating lease. |
| ● | IFRS 16 includes two exceptions which allow lessees to account for leases based on the existing accounting treatment for operating leases - leases for which the underlying asset is of low financial value and short-term leases (up to one year). |
IFRS 16 is effective for annual periods beginning on or after January 1, 2019.
IFRS 16 permits lessees to use one of the following approaches:
| 1. | Full retrospective approach - according to this approach, a right-of-use asset and the corresponding liability will be presented in the statement of financial position as if they had always been measured according to the provisions of IFRS 16. Accordingly, the effect of the adoption of IFRS 16 at the beginning of the earliest period presented will be recorded in equity. Also, we will restate the comparative data in its financial statements. Under this approach, the balance of the liability as of the date of initial application of IFRS 16 will be calculated using the interest rate implicit in the lease, unless this rate cannot be easily determined in which case the lessee’s incremental borrowing rate of interest on the commencement date of the lease will be used. |
| 2. | Modified retrospective approach - this approach does not require restatement of comparative data. The balance of the liability as of the date of initial application of IFRS 16 will be calculated using the lessee’s incremental borrowing rate of interest on the date of initial application of IFRS 16. As for the measurement of the right-of-use asset, we may choose, on a lease-by-lease basis, to apply one of the two following alternatives: |
| ● | Recognize an asset in an amount equal to the lease liability, with certain adjustments. |
| ● | Recognize an asset as if the new standard had always been applied. |
Any difference arising on the date of first-time is recorded in equity.
We believe, based on an assessment of the impact of the adoption of IFRS 16, that its application is not expected to have a material effect on the financial statements.
Recent Offerings
On September 21, 2015, we sold to certain institutional investors providing for the issuance of an aggregate of 2,068,966 ADSs in a registered direct offering at $4.35 per ADS resulting in gross proceeds of $9,000,002. In addition, we issued to the investors unregistered warrants to purchase 1,034,483 ADSs in a private placement. The warrants may be exercised after six months from issuance for a period of five and a half years from issuance and have an exercise price of $5.25 per ADS, subject to adjustment as set forth therein. The warrants may be exercised on a cashless basis if six months after issuance there is no effective registration statement registering our ADSs underlying the warrants. We paid an aggregate of $792,379 in placement agent fees and expenses and issued unregistered placement agent warrants to purchase 103,448 ADS on the same terms as the warrants except they have a term of five years.
On October 15, 2015, we sold to certain institutional investors providing for the issuance of an aggregate of 1,109,196 ADSs in a registered direct offering at $4.35 per ADS resulting in gross proceeds of approximately $4,825,000. In addition, we issued to the investors unregistered warrants to purchase 443,678 ADSs in a private placement. The warrants may be exercised after six months from issuance for a period of five and a half years from issuance and have an exercise price of $5.25 per ADS, subject to adjustment as set forth therein. The warrants may be exercised on a cashless basis if six months after issuance there is no effective registration statement registering our ADSs underlying the warrants. We paid an aggregate of $524,621 in placement agent fees and expenses and issued unregistered placement agent warrants to purchase 55,460 ADS on the same terms as the warrants except they have a term of five years.
84
On January 24, 2017, we sold to certain institutional investors providing for the issuance of an aggregate of 2,500,000 ADSs in a registered direct offering at $2.00 per ADS resulting in gross proceeds of $5,000,000. In addition, we issued to the investors unregistered warrants to purchase 1,250,000 ADSs in a private placement. The warrants may be exercised after six months from issuance for a period of five and a half years from issuance and have an exercise price of $2.25 per ADS, subject to adjustment as set forth therein. The warrants may be exercised on a cashless basis if six months after issuance there is no effective registration statement registering our ADSs underlying the warrants. We paid an aggregate of $360,000 in placement agent fees and expenses and issued unregistered placement agent warrants to purchase 125,000 ADS on the same terms as the warrants except they have a term of five years.
On March 13, 2018, we sold to certain institutional investors providing for the issuance of an aggregate of 3,333,336 ADSs in a registered direct offering at $1.50 per ADS resulting in gross proceeds of approximately $5,000,000. In addition, we issued to the investors unregistered warrants to purchase 2,500,002 ADSs in a private placement. The warrants may be exercised after six months from issuance for a period of five and a half years from issuance and have an exercise price of $2.00 per ADS, subject to adjustment as set forth therein. The warrants may be exercised on a cashless basis if six months after issuance there is no effective registration statement registering our ADSs underlying the warrants. We paid an aggregate of $350,000 in placement agent fees and expenses and issued unregistered placement agent warrants to purchase 166,667 ADS on the same terms as the warrants except they have a term of five years.
On January 18, 2019, we sold to a single institutional investor an aggregate 2,238,096 ADSs in a registered direct offering at $1.05 per ADS, resulting in gross proceeds of $2,350,000. In addition, we issued to the investor unregistered warrants to purchase 2,238,096 ADSs in a private placement. The warrants are immediately exercisable from the date of issuance for a period of five and a half years and have an exercise price of $1.30 per ADS, subject to adjustment as set forth therein. The warrants may be exercised on a cashless basis if six months after issuance there is no effective registration statement registering the ADSs underlying the warrants. We paid an aggregate of $191,000 in placement agent fees and expenses and issued unregistered placement agent warrants to purchase 111,905 ADS on the same terms as the warrants except they have a term of five years.
Jumpstart Our Business Startups Act of 2012
We are an emerging growth company within the meaning of the rules under the Securities Act, and we will utilize certain exemptions from various reporting requirements that are applicable to public companies that are not emerging growth companies. The JOBS Act permits us, as an “emerging growth company,” to take advantage of an extended transition period to comply with certain new or revised accounting standards if such standards apply to companies that are not issuers. We are choosing to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards when they are required to be adopted by issuers. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.
A. Results of Operations
Comparison of the Year Ended December 31, 2018 to Year Ended December 31, 2017
Revenues
Revenues for the year ended December 31, 2018 were $3.8 million, an increase of $3.0 million, or 384%, compared to $0.8 million for the year ended December 31, 2017. The increase in revenue was mainly due to the recognition of a $2 million advance payment received in August 2018 under the Distribution Agreement with CMS Medical and from the recognition of a portion of the $2.2 million advance payment received in January 2018 under the Distribution and Supply Agreement with Gebro.
85
Research and development expenses
Research and development expenses for the year ended December 31, 2018 were $6.0 million, an increase of $0.9 million, or 19%, compared to $5.1 million for the year ended December 31, 2017. Research and developments expenses for the year ended 2018 comprised primarily of expenses associated with the Phase II studies for Namodenoson as well as expenses for ongoing studies of Piclidenoson. The increase is primarily due to increased costs associated with the initiation of the Phase III clinical trial of Piclidenoson for the treatment of rheumatoid arthritis. We expect that the research and development expenses will increase through 2019 and beyond.
General and administrative expenses
General and administrative expenses were $3.1 million for the year ended December 31, 2018 an increase of $0.3 million, or 10%, compared to $2.8 million for the year ended December 31, 2017. The increase is primarily due to an increase in professional services and investor relations expenses. We expect that general and administrative expenses will remain at the same level through 2019.
Financial expenses, net
Financial expenses, net for the year ended December 31, 2018 aggregated $1.1 million compared to immaterial financial income, net for the same period in 2017. The increase in financial expense, net was mainly due to a loss from long-term investment revaluation and from recognition of interest expenses related to implementation of revenue recognition accounting standard IFRS 15, while in the same period in 2017, financial income was mainly due to fair value revaluation of warrants which were offset by financial expenses from exchange rate differences.
Comparison of the Year Ended December 31, 2017 to Year Ended December 31, 2016
Revenues
Revenues for the year ended December 31, 2017 were $0.8 million, an increase of $0.6 million, or 300%, compared to $0.2 million for the year ended December 31, 2016. The revenues during 2017 were mainly due to recognition of a portion of the $0.2 million advance payment received in March 2015 under the Distribution and Supply Agreement with Cipher and from the recognition of the milestone payment of $0.5 million and a portion of the $0.1 million advance payment received in December 2016 under the Distribution Agreement with CKD.
Research and development expenses
Research and development expenses for the year ended December 31, 2017 were $5.1 million, a decrease of $1.0 million, or 16%, compared to $6.1 million for the year ended December 31, 2016. Research and developments expenses for the year ended 2017 comprised primarily of expenses associated with the Phase II studies for Namodenoson as well as expenses for ongoing studies of Piclidenoson. The decrease is primarily due to costs associated with CF602 expenses that decreased since the postponement of a planned IND submission for this indication and a decrease in costs associated with the ongoing clinical trial of Namodenoson for treatment in liver cancer.
General and administrative expenses
General and administrative expenses were $2.8 million for the year ended December 31, 2017, an increase of $0.1 million, or 4.5%, compared to $2.7 million for the year ended December 31, 2016. The minor increase is primarily due to an increase in salary and related expenses.
Financial expenses, net
Financial income, net for the year ended December 31, 2017 aggregated $0.01 million compared to financial income, net of $0.3 million for the same period in 2016. The decrease in financial income, net was mainly due to an increase in financial expenses from exchange rate offset by a decrease in fair value revaluation of warrants.
86
Comparison of the Year Ended December 31, 2016 to Year Ended December 31, 2015
Revenues
Revenues for the year ended December 31, 2016 were $0.2 million compared to $0.2 million for the year ended December 31, 2015. The revenues during 2016 were mainly due to the recognition of a portion of the $1.3 million (CAD 1.65 million) advance payment received in March 2015 under the Distribution and Supply Agreement with Cipher and a minor amount due to the recognition of a portion of the $0.5 million advance payment received in December 2016 under the Distribution Agreement with CKD.
Research and development expenses
Research and development expenses for the year ended December 31, 2016 were $6.1 million, an increase of $2.2 million, or 56%, compared to $3.9 million for the year ended December 31, 2015. Research and developments expenses for the year ended 2016 comprised primarily of expenses associated with the Phase II study for Namodenoson as well as expenses for ongoing studies of Piclidenoson. The increase is primarily due to costs associated with preparations of the Piclidenoson Phase III studies in the treatment of rheumatoid arthritis and psoriasis and costs associated with the ongoing clinical trial of Namodenoson for treatment in liver cancer.
General and administrative expenses
General and administrative expenses were $2.7 million for the year ended December 31, 2016 compared to $2.7 million for the year ended December 31, 2015. The increase considered immaterial.
Financial expenses, net
Financial income, net for the year ended December 31, 2016 aggregated $0.3 million compared to financial expense, net of $0.02 million for the same period in 2015. The increase in financial income, net in the year ended December 31, 2016 considered immaterial.
B. Liquidity and Capital Resources
Since inception, we have funded our operations primarily through public (in Israel and the United States) and private offerings of our equity securities and payments received under our strategic licensing arrangements. As of December 31, 2018, we had approximately $3.6 million in cash and cash equivalents, and have invested most of our available cash funds in ongoing cash accounts. In January 2019, we raised $2.35 million in a registered direct offering and concurrent private placement.
We may be able to use U.S. taxes withheld as credits against Israeli corporate income tax when we have income, if at all, but there can be no assurance that we will be able to realize the credits. In addition, we believe that we may be entitled to a refund of such withholding tax from the U.S. government but there can be no assurance that we will be entitled to such a refund. For information regarding the revenues and expenses associated with our licensing agreements, see “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”, “Item 4. Information on the Company—B. Business Overview—In-Licensing Agreements” and “Item 5. Operating and Financial Review and Prospects—Revenues.”
Net cash used in operating activities was $4.1 million for the year ended December 31, 2018, compared with net cash used in operating activities of $8.9 million and $8.7 million for the years ended December 31, 2017 and 2016, respectively. The $4.8 million decrease in the net cash used in operating activities during 2018, compared to 2017, was primarily the result of an increase in accounts receivable, prepaid expenses and lease deposit, an increase in deferred revenues and a change in fair value of short-term investment. The $0.2 million increase in the net cash used in operating activities during 2017, compared to 2016, is immaterial.
Net cash used in investing activities for the year ended December 31, 2018 was $0.03 million compared to net cash used in investing activities of $0.03 million for the year ended December 31, 2017 and net cash used in investing activities of $0.01 million for the year ended December 31, 2016. The changes in cash flows from investing activities are immaterial.
87
Net cash provided by financing activities for the year ended December 31, 2018 was $4.4 million, compared to $4.5 million for the year ended December 31, 2017 and no net cash provided by financing activities for the year ended December 31, 2016. Net cash provided by financing activities during 2018 and 2017 was due to issuance of shares and warrants, net of issuance expenses, and the decrease of $0.1 million compared to 2017 is immaterial. In January 2017, we raised gross proceeds of $5.0 million in a registered direct offering, and in March 2018, we raised gross proceeds of approximately $5 million in a registered direct offering. The $4.5 million increase in the net cash provided by financing activities during 2017, compared to 2016, was primarily due to issuance of shares and warrants, net of issuance expenses.
Developing drugs, conducting clinical trials and commercializing products is expensive and we will need to raise substantial additional funds to achieve our strategic objectives. Although we believe our existing financial resources as of the date of issuance of this Annual Report on Form 20-F, will be sufficient to fund our projected cash requirements at least through the next twelve months, we will require significant additional financing to fund our operations. Additional financing may not be available on acceptable terms, if at all. Our future capital requirements will depend on many factors, including:
| ● | the level of research and development investment required to develop our product candidates; | |
| ● | the failure to obtain regulatory approval or achieve commercial success of our product candidates, including Piclidenoson, Namodenoson and CF602; |
| ● | the results of our preclinical studies and clinical trials for our earlier stage product candidates, and any decisions to initiate clinical trials if supported by the preclinical results; |
| ● | the costs, timing and outcome of regulatory review of our product candidates that progress to clinical trials; |
| ● | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our issued patents and defending intellectual property-related claims; |
| ● | the cost of commercialization activities if any of our product candidates are approved for sale, including marketing, sales and distribution costs; |
| ● | the cost of manufacturing our product candidates and any products we successfully commercialize; |
| ● | the timing, receipt and amount of sales of, or royalties on, our future products, if any; |
| ● | the expenses needed to attract and retain skilled personnel; |
| ● | any product liability or other lawsuits related to our products; |
| ● | the extent to which we acquire or invest in businesses, products or technologies and other strategic relationships; |
| ● | the costs of financing unanticipated working capital requirements and responding to competitive pressures; and | |
| ● | maintaining minimum shareholders’ equity requirements under the NYSE American Company Guide. |
Until we can generate significant continuing revenues, we expect to satisfy our future cash needs through payments received under our license agreements, debt or equity financings, or by out-licensing other product candidates. We cannot be certain that additional funding will be available to us on acceptable terms, or at all. If funds are not available, we may be required to delay, reduce the scope of, or eliminate one or more of our research or development programs or our commercialization efforts.
88
Research and Development, Patents and Licenses, Etc.
For information concerning our research and development policies and a description of the amount spent during each of the last three fiscal years on company-sponsored research and development activities, see “Item 5. Operating and Financial Review and Prospects— Results of Operation.”
Trend Information.
We are a development stage company and it is not possible for us to predict with any degree of accuracy the outcome of our research, development or commercialization efforts. As such, it is not possible for us to predict with any degree of accuracy any significant trends, uncertainties, demands, commitments or events that are reasonably likely to have a material effect on our net sales or revenues, income from continuing operations, profitability, liquidity or capital resources, or that would cause financial information to not necessarily be indicative of future operating results or financial condition. However, to the extent possible, certain trends, uncertainties, demands, commitments and events are identified in the preceding subsections of this Operating and Financial Review and Prospects.
Off-Balance Sheet Arrangements.
We have no off-balance sheet arrangements that have had or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Contractual Obligations.
The following table summarizes our significant contractual obligations in U.S. dollars as of December 31, 2018:
| Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | ||||||||||||||||
| Contractual Obligations | ||||||||||||||||||||
| NIH milestones(1) | 425,000 | 425,000 | - | - | - | |||||||||||||||
| Leiden University milestones(2) | 91,603 | 11,450 | 80,153 | - | - | |||||||||||||||
| Car lease obligations | 30,048 | 19,709 | 10,339 | - | - | |||||||||||||||
| Total | 564,651 | 456,159 | 90,492 | - | - | |||||||||||||||
| (1) | Includes $425,000 in milestone payments. |
| (2) | Includes a €10,000 annual royalty and €50,000 upon the initiation of a Phase I study. We will update our milestone payment obligations upon releasing the Phase I data from such study. As such, the obligations above do not include a potential milestone payment of €100,000 upon the initiation of a Phase II study, €200,000 upon the initiation of a Phase III study or €500,000 upon marketing approval by any regulatory authority. |
Other than as described above, we did not have any material commitments for capital expenditures, including any anticipated material acquisition of plant and equipment or interests in other companies, as of December 31, 2018.
89
ITEM 6. Directors, Senior Management and Employees
A. Directors and Senior Management.
The following table sets forth our directors and senior management:
| Member | Age | Position | ||
| Ilan Cohn, Ph.D. | 63 | Chairman of the Board | ||
| Pnina Fishman, Ph.D. | 70 | Chief Executive Officer, Director | ||
| Motti Farbstein | 55 | Chief Operating and Financial Officer | ||
| Sari Fishman, Ph.D. | 47 | VP of Business Development | ||
| Guy Regev | 49 | Director, Audit Committee and Compensation Committee member | ||
| Abraham Sartani, M.D. | 72 | Director | ||
| Israel Shamay | 54 | Director, Audit Committee and Compensation Committee member | ||
| Yaacov Goldman | 63 | Director, Audit Committee and Compensation Committee member |
Ilan Cohn, Ph.D. Ilan Cohn, Ph.D. is a patent attorney and senior partner at the patent attorney firm Reinhold Cohn and Partners, where he has been an attorney since 1986. Dr. Cohn co-founded Can-Fite, served as its Chief Executive Officer until September 2004, served on our Board of Directors since 1994 and since May 30, 2013 serves as the Chairman of the Can-Fite Board of Directors. Dr. Cohn has also been a director of OphthaliX since November 21, 2011. Dr. Cohn holds a Ph.D. in biology and is a patent attorney with many years of experience in the biopharmaceutical field. He has served on the Board of Directors of a number of life science companies, including Discovery Laboratories Inc. (formerly Ansan Pharmaceuticals), a U.S. public company. Dr. Cohn has also been involved in the past in management of venture capital funds focused on investments in the life sciences industry. Dr. Cohn served a number of years as a co-chairman of the Biotech Committee of the US-Israeli Science and Technology Commission. Dr. Cohn is also currently a member of the Board of Directors of I.C.R.C. Management Ltd, Famillion BVI Ltd. and Famillion Ltd. (a subsidiary of Famillion BVI Ltd.). Dr. Cohn holds a Ph.D. in Biology from the Hebrew University of Jerusalem.
Pnina Fishman, Ph.D. Pnina Fishman, Ph.D. co-founded Can-Fite and has served as our Chief Executive Officer and served on our Board of Directors since September 2005. Dr. Fishman is the scientific founder of Can-Fite and was previously a professor of Life Sciences and headed the Laboratory of Clinical and Tumor Immunology at the Felsenstein Medical Research Institute, Rabin Medical Center, Israel. Dr. Fishman has authored or co-authored over 150 publications and presented the findings of her research at many major scientific meetings. Her past managerial experience included seven years as Chief Executive Officer of Mor Research Application, the technology transfer arm of Clalit Health Services, the largest healthcare provider in Israel. Mor Research Application was also the first clinical research organization in Israel. Dr. Fishman currently also serves as a member of the Board of Directors of F.D Consulting Ltd., Ultratrend Ltd., and Eye-Fite Ltd. Dr. Fishman holds a Ph.D. in Immunology from the Bar Ilan University in Ramat Gan, Israel.
Motti Farbstein. Motti Farbstein has been with Can-Fite since 2003. Mr. Farbstein served as our Chief Operating Officer from August 2003 until May 2005 and from that date onwards he served as Chief Operating and Financial Officer. Mr. Farbstein also serves as a director of Eye-Fite Ltd. since July 2011. Mr. Farbstein’s past managerial experience includes seven years as Vice President of Mor Research Application, a company that managed the commercialization of the intellectual property of all hospitals and research centers affiliated with Clalit Health Services, which is the largest healthcare provider in Israel and was Israel’s first clinical CRO. Mr. Farbstein also has extensive experience in the data management of clinical trials.
90
Sari Fishman, Ph.D. Sari Fishman, Ph.D. has served as our Director Clinical Affairs from 2004 to 2014, Director of Business Development from 2014 to 2017 and since 2017 serves as VP of Business Development. Dr. Fishman gained her Ph.D. at the Bar-Ilan University, Ramat-Gan, Israel.
Abraham Sartani, M.D. Abraham Sartani has served on our Board of Directors since 2001. Dr. Sartani has over 30 years of experience in the pharmaceuticals industry and currently acts as a consultant to pharmaceutical and medical device companies. Dr. Sartani is a member of a number of scientific and management societies and the author or co-author of numerous publications and patents in the urology, pain treatment and hypertension fields. Dr. Sartani previously served on the Board of Directors of Akkadeas Pharma Srl (formerly Arkadia Pharma) and was a co-founder. From 1985 until 2008, Dr. Sartani was the Vice-President of R&D and Licensing and Group coordinator of B&D of Recordati, a European specialty pharmaceutical company. Prior to joining Recordati, from 1980 until 1985, Dr. Sartani was employed at Farmitalia-Carlo Erba, serving in a number of capacities, including as the Medical Director for Europe.
Guy Regev. Guy Regev has over twelve years of experience in accounting, financial management and control and general management of commercial enterprises. He has served on our Board of Directors since July 2011 and has served as a member of our Audit Committee and Compensation Committee since February 2014. Mr. Regev has also been a director of OphthaliX since November 2011. Mr. Regev is currently the Chief Executive Officer of Gaon Holdings Ltd, a publicly traded Israeli holding company traded on the TASE which focuses on three areas of operation - Cleantech / Water, Financial Services, Retail/Trading. Mr. Regev is currently also the Chief Executive Officer of Middle East Tube Company Ltd a publicly traded Israeli company traded on the TASE which focuses on steel pipe manufacturing and galvanization services. Mr. Regev was the Chief Executive Officer of Shaked Global Group Ltd, a privately-held equity investment firm that provides value added capital to environmental-related companies and technologies. Prior to joining Shaked, from 2001 to 2008, Mr. Regev was Vice President of Commercial Business at Housing & Construction Holding, or HCH, Israel’s largest infrastructure company. His duties included being responsible for the consolidation and financial recovery of various business units within HCH. Prior to that, Mr. Regev carried several roles within the group including as a Chief Financial Officer and later the Chief Executive Officer of Blue-Green Ltd., the environmental services subsidiary of HCH. Between 1999 and 2001, Mr. Regev was a manager at Deloitte & Touche, Israel. Mr. Regev holds an LLB degree in Law (Israel) and is a licensed attorney and has been a licensed CPA since 1999. Mr. Regev is also a director of, The Green Way Ltd, Shtang Construction and Engineering Ltd, R.I.B.E. Consulting & Investment Ltd., Middle East Tube Company Ltd, Middle East Tube - Industries 2001 Ltd, Middle East Tubes - Galvanizing (1994) Ltd, I-Solar Greentech Ltd, Plassim Infrastructure Ltd, Plassim Advanced Solutions in Sanitation Ltd, Hakohav Valves Industries Metal (1987) Ltd, Metzerplas Agriculture Cooperative Ltd, B. Gaon Retail & Trading Ltd, Gaon Agro - Rimon Management Services Ltd, B. Gaon Business (2004) Ltd, Gaon Antan Investments Ltd, Or Asaf Investments Ltd, Hamashbir Holdings (1999) Ltd, and AHAVA Holdings LTD.
Israel Shamay. Israel Shamay has served as external director since December 2014 and serves as a member on both the Audit Committee and Compensation Committee. Since 2012 Mr. Shamay has served as Executive Director, Strategic Initiatives and Head of the Americas Operations of MATIMOP (Israeli Industry Center for R&D), the International Operations agency of the Israeli Office of the Israel Innovation Authority (formerly the Office of Chief Scientist), focusing on developing and implementing cooperation platforms for industrial R&D and innovation projects in the Americas region. From 2006 until 2012 Mr. Shamay served as Executive Director of European Cooperations at MATIMOP, where he was in charge of architecting, realizing and evaluating industrial innovation cooperation frameworks at bilateral and European level, making them a major R&D cooperation instrument for Israeli industry with Europe. Between 2010 and 2011, Mr. Shamay was Head of the Israeli EUREKA Chairmanship Program (EUREKA is Europe’s largest innovation network with nearly 40 member states). The Israeli EUREKA Chairmanship focused on developing new financial instruments for innovative small and medium sized enterprises and on expanding EUREKA’s international dimension. From 2002 Mr. Shamay served as Israel’s National Representative in several international R&D programs, from 2005 as an expert evaluator for the EU Framework Programs for R&D and from 2006 until 2009 managed the Israeli R&D collaboration with the EU Global Satellite Navigation Program – GALILEO. From 1991 until 2001, Mr. Shamay served in senior technical, marketing and executive positions in Israeli hi-tech companies operating globally, including the RAD group and Comverse Technologies. Mr. Shamay is an MBA graduate of the Recanati School of Business at the Tel-Aviv University and a graduate of the Technion in Haifa, faculty of Information Systems Engineering.
91
Yaacov Goldman. Yaacov Goldman has served as external director since August 2017. Mr. Goldman provides consulting services to companies in strategic-financial areas, through his wholly owned company, Maanit-Goldman Management & Investments (2002) Ltd. Mr. Goldman also serves as a director of Avgol Industries 1953 Ltd., Meitav Dash Investments Ltd., Industrial Buildings Corporation Ltd., IceCure Medical Ltd. and Fattal Properties (Europe) Ltd. Mr. Goldman served as the Professional Secretary of the Peer Review Institute of the Certified Public Accountants Institute in Israel from October 2004 until September 2008. Commencing in 1981, Mr. Goldman worked for Kesselman & Kesselman (Israeli member firm of PricewaterhouseCoopers) for 19 years, and from 1991 until 2000, as a partner and then senior partner of such firm. From September 2000 until November 2001, Mr. Goldman served as managing director of Argoquest Holdings, LLC. Mr. Goldman holds a B.A. degree in Economics and Accounting from Tel Aviv University and is a Certified Public Accountant (Israel).
B. Compensation.
Compensation of Directors and Senior Management
The following table presents in the aggregate all compensation we paid to all of our office holders as a group for the year ended December 31, 2018. It does not include any business travel, relocation, professional, and business association dues and expenses reimbursed to office holders, and other benefits commonly reimbursed or paid by companies in Israel.
The term ‘office holder’ as defined in the Companies Law includes a general manager, chief business manager, deputy general manager, vice general manager, any other person fulfilling or assuming the responsibilities of any of the foregoing positions without regard to such person’s title, as well as a director, or a manager directly subordinate to the general manager or the chief executive officer. As of March 21, 2018, in addition to the six members of the Board of Directors (including the Company’s Chief Executive Officer), the Company considers two other individuals, including its Chief Financial Officer and its VP Business Development to be office holders.
| Salaries, fees, commissions, bonuses and options (thousand NIS) | ||||
| All office holders as a group, consisting of 8 persons | 3,024 | |||
92
The following table presents information regarding compensation reflected in our financial statements for five most highly compensated office holders, as of December 31, 2018.
| Name and Position | Salary | Bonus(4) | Value of Options Granted(5) | Other(6) | Total | |||||||||||||||
| (NIS in thousands) | ||||||||||||||||||||
| Pnina Fishman Chief Executive Officer | 1,301 | (1) | 337 | 57 | 50 | 1,745 | ||||||||||||||
| Motti Farbstein Chief Financial Officer | 694 | (2) | 240 | 255 | 50 | 1,239 | ||||||||||||||
| Sari Fishman VP Business Development | 529 | (2) | 200 | 212 | 50 | 991 | ||||||||||||||
| Yaacov Goldman External Director | 112 | (3) | - | 37 | - | 149 | ||||||||||||||
| Guy Regev External Director | 108 | (3) | - | 37 | - | 145 | ||||||||||||||
| (1) | Amount represents consulting fee. |
| (2) | Salary includes gross salary plus payment of social benefits made by us on behalf of such person. Such benefits may include, to the extent applicable, payments, contributions and/or allocations for savings funds (e.g., managers’ life insurance policy), education funds (referred to in Hebrew as “keren hishtalmut”), pension, severance, risk insurances (e.g., life, or work disability insurance), payments for social security payments and tax gross-up payments, vacation, medical insurance and benefits, convalescence or recreation pay and other benefits and perquisites consistent with our policies. |
| (3) | Amount represents fees for board service. |
| (4) | Amounts reported in this column refer to the cash bonuses provided by us with respect to 2018, which have been provided for in our financial statements for the year ended December 31, 2018 (including if such bonuses were paid in 2019). They exclude bonuses paid in 2018 which were provided for in the Company’s financial statements for previous years. |
| (5) | The value of options is the expense recorded in our financial statements for the period ended December 31, 2018 with respect to all options granted to such person. Assumptions and key variables used in the calculation of such amounts are discussed in Note 12 of our financial statements. |
| (6) | Amount represents cost of use of company car. |
Each director other than our Chief Executive Officer and Avraham Sartani, is entitled to the payment of annual fee of NIS 48,721 (approximately $12,671), and payment of NIS 3,256 (approximately $847) per meeting for participating in meetings of the board and committees of the board. The annual fee shall not exceed the annual fee of an expert external director set forth in the Companies Regulations (Rules regarding Compensation and Expenses of External Directors) 5760-2000 as adjusted by the Companies Regulations (Relief for Public Companies with Shares Listed for Trading on a Stock Market Outside of Israel), 5760-2000. The compensation awarded for participating in resolutions that are adopted without an actual convening (i.e., unanimous written resolutions) and for participating through telephone meetings will be reduced as follows: (1) for resolutions that will be adopted without an actual convening, the participation compensation will be reduced by 50%; and (2) for participation through telephone meetings, the participation compensation will be reduced by 40%. The participation compensation and the annual fee is inclusive of all expenses incurred by our directors in connection with their participation in a meeting held at our offices or with regard to resolutions resolved by written consent or teleconference. Avraham Sartani is entitled to a fee of $1,000 per meeting. In addition, our directors (other than our Chief Executive Officer and external directors) are entitled to reimbursement for expenses related to their participation at meetings taking place not at our offices and outside their respective residency area.
Employment and Consulting Agreements
We have entered into employment or consulting agreements with our directors, senior management and key service providers. All of these agreements contain customary provisions regarding noncompetition, confidentiality of information and assignment of proprietary information and inventions. However, the enforceability of the noncompetition provisions may be limited under applicable law.
93
The following are summary descriptions of certain agreements to which we are a party. The descriptions provided below do not purport to be complete and are qualified in their entirety by the complete agreements, which are attached as exhibits to this Annual Report on Form 20-F.
Service Management Agreement with F.D. Consulting: On June 27, 2002, we entered into a Service Management Agreement with F.D. Consulting, a company partially owned by Pnina Fishman, pursuant to which Dr. Fishman began serving as our Chief Scientific Officer and later became our Chief Executive Officer and is a member of our Board of Directors and continues to be retained through this agreement. F.D. Consulting’s current gross monthly fee is NIS 108,360 which is linked to the Israeli CPI and fluctuates accordingly. Dr. Fishman, through F.D. Consulting, is also entitled to reimbursement for reasonable out-of-pocket expenses and use of a company automobile and mobile phone.
The term of F.D. Consulting’s service management agreement is indefinite, unless earlier terminated for cause by us or without cause by either party, subject to three months’ advanced notice.
Dr. Fishman is also entitled to receive options exercisable into our ordinary shares from time to time. As of March 21, 2019, we have granted her options to purchase an aggregate of 744,443 ordinary shares, of which (i) 241,613 were exercised into ordinary shares, (ii) options to purchase 195,630 ordinary shares expired, (iii) 2,680,000 options to purchase 107,200 ordinary shares have an exercise price of NIS 0.644 per option, are fully vested and expire on January 13, 2021, and (iii) 200,000 options to purchase 200,000 ordinary shares have an exercise price of NIS 3.573 per ordinary share, vesting on a quarterly basis over three years commencing October 22, 2015, and expire on October 22, 2025.
On January 7, 2019, our compensation committee and board of directors approved the grant, subject to shareholder approval which was obtained on March 11, 2019, to Dr. Fishman of 400,000 options to purchase 400,000 ordinary shares of the Company. The options will be issued under the following terms: (i) the exercise price per each such option shall be an exercise price equal to the average price of our ordinary shares on the Tel Aviv Stock Exchange in the 30 trading days before the issuance; and (ii) such options shall vest on a quarterly basis over four years such that 25,000 options shall vest at the end of each calendar quarter and that the options shall be granted in accordance with our 2013 Share Option Plan.
Employment and Non-Competition Agreement with Motti Farbstein: On September 1, 2003 we entered into an employment and non-competition agreement with Motti Farbstein pursuant to which Mr. Farbstein began serving as our Director of Clinical Operations and Administrative Affairs on September 1, 2003 and is currently serving as our Chief Operating and Financial Officer. Mr. Farbstein’s current gross monthly salary is NIS 52,000. Mr. Farbstein is entitled to an allocation to a manager’s insurance policy equivalent to an amount up to 13-1/3% of his gross monthly salary, up to 2-1/2% of his gross monthly salary for disability insurance and 7-1/2% of his gross monthly salary for a study fund. The foregoing amounts are paid by us. Five percent of his gross monthly salary is deducted for the manager’s insurance policy and 2-1/2% is deducted for the study fund. Mr. Farbstein is also entitled to reimbursement for reasonable out-of-pocket expenses, including travel expenses, and use of a company automobile and mobile phone.
The term of Mr. Farbstein’s employment and non-competition agreement is indefinite, unless earlier terminated for just cause by either party, upon the death, disability or retirement age, or without cause by either party, subject to 60 days’ advanced notice.
Mr. Farbstein is also entitled to receive options exercisable into our ordinary shares from time to time. As of March 21, 2019, we have granted him options to purchase an aggregate of 514,195 ordinary shares, of which (i) options to purchase ordinary shares were exercised into 1,133 ordinary shares, (ii) options to purchase 35, 062 ordinary shares, (iii) 100,000 options are exercisable into 4,000 ordinary shares at an exercise price of NIS 0.385 per option, are fully vested, and expire on May 2, 2022, (iv) 100,000 options are exercisable into 4,000 ordinary shares at an exercise price of NIS 0.326 per option are fully vested, and expire on March 20, 2023, (v) 10,000 options to purchase 10,000 ordinary shares at an exercise price of NIS 8.1205 per option, vesting on a quarterly basis over four years commencing March 19, 2015, and expire on March 18, 2025, (vi) 60,000 options to purchase 60,000 ordinary shares at an exercise price of NIS 4.317 per option, vesting on a quarterly basis over four years commencing February 18, 2016 and expire on February 18, 2026, (vii) 250,000 options to purchase 250,000 ordinary shares at an exercise price of NIS 2.513 per option, vesting on a quarterly basis over four years commencing December 28, 2017 and expire on December 28, 2027, and (viii) 150,000 options to purchase 150,000 ordinary shares at an exercise price of NIS 2.344 per option, vesting on a quarterly basis over four years commencing January 7, 2019 and expire on January 7, 2029.
94
Consulting Agreement with BioStrategics: On September 27, 2005, we entered into a consulting agreement with BioStrategics through its President, Michael Silverman pursuant to which Dr. Silverman began serving as our Medical Director. Dr. Silverman has extensive experience in clinical development acquired through his involvement in clinical development in large pharmaceutical and small biopharmaceutical companies. He was involved in international clinical research, market-oriented strategic planning, and the challenges of managing research and development portfolios in various capacities at Sterling Winthrop Research Institute and subsequently at Sandoz Research Institute.
BioStrategics’ current fee is $400 per hour with a maximum daily fee of $2,600. In addition, BioStrategics is entitled to reimbursement for reasonable pre-approved expenses. The term of the consulting agreement is currently on a year-to-year basis, unless earlier terminated by either party upon 30 days’ prior written notice or immediately by either party if such termination is for cause.
Master Services Agreement with Accellient Partners: On May 10, 2010, we entered into a Master Services Agreement with Accellient Partners, a company owned by William Kerns, who currently serves as our current Vice President of Drug Development. Dr. Kerns has over 20 years of experience in Pharmaceutical Research and Development at SmithKline Beecham and Eisai Pharmaceuticals. As a Senior Executive he has participated in the development of drugs for over 100 Phase I studies and 13 NDA’s and/or Marketing Authorization Applications. Dr. Kerns has chaired a FDA committee on biomarkers and he is an expert in preclinical development and regulatory strategy.
According to the agreement, consulting services are provided by Accellient Partners’ personnel in accordance with individual work orders that are executed from time to time. Each individual work order defines the scope of work to be provided and sets forth the fees to be paid to Accellient Partners.
Beginning on May 10, 2012, the term of the master services agreement is on a month-to-month basis, unless terminated by us upon 30 days’ prior written notice, by us at any time if Accellient Partners commits a breach and fails to cure, or by Accellient Partners upon 30 days’ prior written notice if we commit a breach and fail to cure.
Reinhold Cohn and Partners: Reinhold Cohn and Partners, an Israeli partnership, of which Ilan Cohn, Ph.D. is a partner provides intellectual property services to us in the ordinary course of business.
C. Board Practices
General
According to the Israeli Companies Law, the management of our business is vested in our Board of Directors. Our Board of Directors may exercise all powers and may take all actions that are not specifically granted to our shareholders. Our executive officers are responsible for our day-to-day management and have individual responsibilities established by our Board of Directors. Executive officers are appointed by and serve at the discretion of our Board of Directors, subject to any applicable employment agreements we have entered into with the executive officers. See “Item 6. Directors, Senior Management and Employees—B. Compensation—Employment and Consulting Agreements.”
Election of Directors and Terms of Office
Our Board of Directors currently consists of six members. Other than our two external directors, our directors are elected by an ordinary resolution at the annual general meeting of our shareholders. The nomination of our directors is proposed by the Board of Directors. Our board has the authority to add additional directors up to the maximum number of 12 directors allowed under our Articles. Such directors appointed by the board serve until the next annual general meeting of the shareholders. Unless they resign before the end of their term or are removed in accordance with our Amended and Restated Articles of Association, all of our directors, other than our external directors, will serve as directors until our next annual general meeting of shareholders. On December 10, 2018, at an annual general meeting of our shareholders, Pnina Fishman, Ilan Cohn, Abraham Sartani, and Guy Regev were re-elected to serve as directors for a term expiring at our next annual general meeting of shareholders and until his or her respective successor is duly elected. On August 1, 2017, at an annual general meeting of our shareholders Yaacov Goldman was elected to serve as one of our external directors for a three-year term ending July 31, 2020. On December 27, 2017, at a special meeting of our shareholders, Israel Shamay was elected to serve for a three-year term ending December 26, 2020 as one of our external directors. Israel Shamay may be re-elected for another three-year term. On May 30, 2013, Ilan Cohn was appointed as Chairman of the Board.
95
None of our directors or senior management has any family relationship with any other director or senior management except that Sari Fishman is the daughter of Pnina Fishman. None of our directors have service contracts that provide for benefits upon termination of his or her directorship with us, other than the payment of salary due, accrued and unpaid as of and through the date of termination. See “Item 6. Directors, Senior Management and Employees—B. Compensation—Employment and Consulting Agreements.”
Chairman of the Board. Under the Israeli Companies Law, without shareholder approval, a person cannot hold the role of both chairman of the board of directors and chief executive officer of a company. Furthermore, a person who is directly or indirectly subordinate to a chief executive officer of a company may not serve as the chairman of the board of directors of that company and the chairman of the board of directors may not otherwise serve in any other capacity in a company or in a subsidiary of that company other than as the chairman of the board of directors of such a subsidiary.
The Israeli Companies Law provides that an Israeli company may, under certain circumstances, exculpate an office holder from liability with respect to a breach of his duty of care toward the company if appropriate provisions allowing such exculpation are included in its articles of association. Our Amended and Restated Articles of Association permit us to maintain directors’ and officers’ liability insurance and to indemnify our directors and officers for actions performed on behalf of us, subject to specified limitations. We maintain a directors and officers insurance policy which covers the liability of our directors and officers as allowed under the Israeli Companies Law.
The term office holder is defined in the Israeli Companies Law as a director, general manager, chief business manager, deputy general manager, vice general manager, executive vice president, vice president, any other manager directly subordinate to the general manager or any other person assuming the responsibilities of any of the foregoing positions, without regard to such person’s title.
External and Independent Directors
Under the Israeli Companies Law, the boards of directors of companies whose shares are publicly traded, either within or outside of Israel, are required to include at least two members who qualify as external directors.
External directors must be elected by a majority vote of the shares present and voting at a shareholders meeting, provided that either:
| ● | the majority of the shares that are voted at the meeting, including at least a majority of the shares held by non-controlling shareholders who do not have a personal interest in the election of the external director (other than a personal interest not deriving from a relationship with a controlling shareholder) who voted at the meeting, excluding abstentions, vote in favor of the election of the external director; or |
| ● | the total number of shares held by non-controlling, disinterested shareholders (as described in the preceding bullet point) that are voted against the election of the external director does not exceed 2% of the aggregate voting rights in the company. |
96
The term controlling shareholder is defined in the Israeli Companies Law as a shareholder with the ability to direct the activities of the Company, other than by virtue of being an office holder, but there is a presumption that a shareholder holding 25% of the shares of the Company is regarded as a controlling shareholder. A person may not serve as an external director of a company if (i) such person is a relative of a controlling shareholder of a company or (ii) at the date of such person’s appointment or within the prior two years, such person, such person’s relative, partner, employer or any entity under such person’s control or anyone to whom such person is subordinate, whether directly or indirectly, has or had any affiliation with (a) the company, (b) the controlling shareholder at the time of such person’s appointment or (c) any entity that is either controlled by the company or under common control with the company at the time of such appointment or during the prior two years. If a company does not have a controlling shareholder or a shareholder who holds company shares entitling him to vote at least 25% of the votes in a shareholders meeting, then a person may not serve as an external director if, such person or such person’s relative, partner, employer or any entity under such person’s control, has or had, on or within the two years preceding the date of the person’s appointment to serve as an external director, any affiliation with the chairman of our board of directors, chief executive officer, a substantial shareholder who holds at least 5% of the issued and outstanding shares of the company or voting rights which entitle him to vote at least 5% of the votes in a shareholders meeting, or the chief financial officer of the company.
The term affiliation includes:
| ● | an employment relationship; |
| ● | a business or professional relationship even if not maintained on a regular basis (excluding insignificant relationships); |
| ● | control; and |
| ● | service as an office holder, excluding service as a director in a private company prior to the first offering of its shares to the public if such director was appointed as a director of the private company in order to serve as an external director following the public offering. |
The term relative is defined as a spouse, sibling, parent, grandparent or descendant; a spouse’s sibling, parent or descendant; and the spouse of each of the foregoing persons.
In addition, no person may serve as an external director if that person’s professional activities create, or may create, a conflict of interest with that person’s responsibilities as a director or otherwise interfere with that person’s ability to serve as an external director or if the person is an employee of the Israel Securities Authority, or the ISA, or of the TASE. Furthermore, a person may not continue to serve as an external director if he or she received direct or indirect compensation from the company for his or her role as a director. This prohibition does not apply to compensation paid or given in accordance with regulations promulgated under the Israeli Companies Law or amounts paid pursuant to indemnification and/or exculpation contracts or commitments and insurance coverage. If, at the time an external director is appointed, all current members of the board of directors not otherwise affiliated with the company are of the same gender, then that external director must be of the other gender. In addition, a director of a company may not be elected as an external director of another company if, at that time, a director of the other company is acting as an external director of the first company.
Following the termination of an external director’s service on a board of directors, such former external director and his or her spouse and children may not be provided with a direct or indirect benefit by the company, its controlling shareholder or any entity under its controlling shareholder’s control. This includes engagement to serve as an executive officer or director of the company or a company controlled by its controlling shareholder, or employment by, or providing services to, any such company for consideration, either directly or indirectly, including through a corporation controlled by the former external director, for a period of two years (and for a period of one year with respect to relatives of the former external director).
97
The Israeli Companies Law provides that an external director must meet certain professional qualifications or have financial and accounting expertise and that at least one external director must have financial and accounting expertise. However, if at least one of our other directors (i) meets the independence requirements of the Securities Exchange Act of 1934, as amended, (ii) meets the standards of the NYSE American rules for membership on the audit committee and (iii) has financial and accounting expertise as defined in the Israeli Companies Law and applicable regulations, then neither of our external directors is required to possess financial and accounting expertise as long as both possess other requisite professional qualifications. Our Board of Directors is required to determine whether a director possesses financial and accounting expertise by examining whether, due to the director’s education, experience and qualifications, the director is highly proficient and knowledgeable with regard to business-accounting issues and financial statements, to the extent that the director is able to engage in a discussion concerning the presentation of financial information in our financial statements, among others. The regulations define a director with the requisite professional qualifications as a director who satisfies one of the following requirements: (i) the director holds an academic degree in either economics, business administration, accounting, law or public administration; (ii) the director either holds an academic degree in any other field or has completed another form of higher education in our primary field of business or in an area which is relevant to the office of an external director; or (iii) the director has at least five years of experience serving in any one of the following, or at least five years of cumulative experience serving in two or more of the following capacities: (a) a senior business management position in a corporation with a substantial scope of business; (b) a senior position in our primary field of business; or (c) a senior position in public administration. Yaacov Goldman, who is one of our external directors, meets the required qualifications and has financial and accounting expertise as required by the Israeli Companies Law, while Guy Regev, an independent director, also meets the required qualifications and has financial and accounting expertise as required by the Israeli Companies Law.
The Israeli Companies Law defines an independent director as a director who complies with the following and was appointed as such in accordance with Chapter 1 of Part 56 of the Israeli Companies Law: (1) the director complies with the qualification to serve as an external director as set out in Sections 240 (b)-(f) of the Israeli Companies Law and the audit committee has approved such compliance; and (2) the director has not served as a director of the company for more than nine consecutive years (which, for such purpose, does not include breaks in such service for periods of less than two year).
If an external directorship becomes vacant and there are less than two external directors on the board of directors at the time, then the board of directors is required under the Israeli Companies Law to call a shareholders’ meeting as soon as possible to appoint a replacement external director.
Each committee of the board of directors that is authorized to exercise the powers of the board of directors must include at least one external director, except that the audit committee and compensation committee must each include all external directors then serving on the board of directors. Under the Israeli Companies Law, external directors of a company are prohibited from receiving, directly or indirectly, any compensation for their services as external directors, other than compensation and reimbursement of expenses pursuant to applicable regulations promulgated under the Israeli Companies Law. Compensation of an external director is determined prior to his or her appointment and may not be changed during his or her term subject to certain exceptions.
Israel Shamay and Yaacov Goldman serve as external directors on our Board of Directors pursuant to the provisions of the Israeli Companies Law. They both serve on our audit committee and our compensation committee. Our Board of Directors has determined that Yaacov Goldman possesses accounting and financial expertise, and that both of our external directors possess the requisite professional qualifications. In addition to our external directors, Guy Regev and Abraham Sartani serve as independent directors on our Board of Directors. Guy Regev also serves on our audit committee and our compensation committee.
Audit Committee
The Israeli Companies Law requires public companies to appoint an audit committee. The responsibilities of the audit committee include identifying irregularities in the management of our business and approving related party transactions as required by law. An audit committee must consist of at least three directors, including all of its external directors and a majority of independent directors. The chairman of the board of directors, any director employed by or otherwise providing services to the company, and a controlling shareholder or any relative of a controlling shareholder, may not be a member of the audit committee. An audit committee may not approve an action or a transaction with a controlling shareholder, or with an office holder, unless at the time of approval two external directors are serving as members of the audit committee and at least one of the external directors was present at the meeting in which an approval was granted.
98
Our audit committee is currently comprised of three independent non-executive directors. The audit committee is chaired by Yaacov Goldman, who serves as the audit committee financial expert, with Israel Shamay and Guy Regev as members. Our audit committee meets at least four times a year and monitors the adequacy of our internal controls, accounting policies and financial reporting. It regularly reviews the results of the ongoing risk self-assessment process, which we undertake, and our interim and annual reports prior to their submission for approval by the full Board of Directors. The audit committee oversees the activities of the internal auditor, sets its annual tasks and goals and reviews its reports. The audit committee reviews the objectivity and independence of the external auditors and also considers the scope of their work and fees.
Our audit committee provides assistance to our Board of Directors in fulfilling its legal and fiduciary obligations in matters involving our accounting, auditing, financial reporting, internal control and legal compliance functions by pre-approving the services performed by our independent accountants and reviewing their reports regarding our accounting practices and systems of internal control over financial reporting. Our audit committee also oversees the audit efforts of our independent accountants and takes those actions that it deems necessary to satisfy itself that the accountants are independent of management.
Under the Israeli Companies Law, our audit committee is responsible for (i) determining whether there are deficiencies in the business management practices of our company, including in consultation with our internal auditor or the independent auditor, and making recommendations to the Board of Directors to improve such practices and amend such deficiencies, (ii) determining whether certain related party transactions (including transactions in which an office holder has a personal interest) should be deemed as material or extraordinary, and to approve such transactions (which may be approved according to certain criteria set out by our audit committee on an annual basis) (see “—Approval of Related Party Transactions under the Israeli Companies Law”); (iii) establishing procedures to be followed in respect of related party transactions with a controlling shareholder (where such are not extraordinary transactions), which may include, where applicable, the establishment of a competitive process for such transaction, under the supervision of the audit committee, or individual, or other committee or body selected by the audit committee, in accordance with criteria determined by the audit committee; (iv) determining procedures for approving certain related party transactions with a controlling shareholder, which having been determined by the audit committee not to be extraordinary transactions, were also determined by the audit committee not to be negligible transactions; (v) approving the working plan of the internal auditor, to examine such working plan before its submission to the Board and proposing amendments thereto, (vi) examining our internal controls and internal auditor’s performance, including whether the internal auditor has sufficient resources and tools to dispose of its responsibilities, (vii) examining the scope of our auditor’s work and compensation and submitting a recommendation with respect thereto to our Board of Directors or shareholders, depending on which of them is considering the appointment of our auditor, and (viii) establishing procedures for the handling of employees’ complaints as to the management of our business and the protection to be provided to such employees.
We have adopted a written charter for our audit committee, setting forth its responsibilities as outlined by the regulations of the SEC. In addition, our audit committee has adopted procedures for the receipt, retention and treatment of complaints we may receive regarding accounting, internal accounting controls or auditing matters and the submission by our employees of concerns regarding questionable accounting or auditing matters. In addition, SEC rules mandate that the audit committee of a listed issuer consist of at least three members, all of whom must be independent, as such term is defined by rules and regulations promulgated by the SEC. We are in compliance with the independence requirements of the SEC rules.
Any person who is not eligible to serve on the audit committee is further restricted from participating in its meetings and votes, unless the chairman of the audit committee determines that such person’s presence is necessary in order to present a certain matter; provided, however, that company employees who are not controlling shareholders or relatives of such shareholders may be present in the meetings, but not for actual voting, and likewise, company counsel and secretary who are not controlling shareholders or relatives of such shareholders may be present in the meetings and for actual voting if such presence is requested by the audit committee.
In addition to the above, all such committee’s members must apply with the following requirements:
| ● | All members shall be members of the board of directors of the company. |
| ● | At least one of the committee’s members shall have financial and accounting expertise and the rest of the committee’s members must have the ability to read and understand financial statements. |
Our company, through our audit committee, is in full compliance with the above requirements.
99
Financial Statement Examination Committee
Under the Israeli Companies Law, the board of directors of a public company must appoint a financial statement examination committee, which consists of members with accounting and financial expertise or the ability to read and understand financial statements. According to a resolution of our Board of Directors, the audit committee has been assigned the responsibilities and duties of a financial statements examination committee, as permitted under relevant regulations promulgated under the Israeli Companies Law. From time to time as necessary and required to approve our financial statements, the audit committee holds separate meetings, prior to the scheduled meetings of the entire Board of Directors regarding financial statement approval. The function of a financial statements examination committee is to discuss and provide recommendations to its board of directors (including the report of any deficiency found) with respect to the following issues: (i) estimations and assessments made in connection with the preparation of financial statements; (ii) internal controls related to the financial statements; (iii) completeness and propriety of the disclosure in the financial statements; (iv) the accounting policies adopted and the accounting treatments implemented in material matters of the company; and (v) value evaluations, including the assumptions and assessments on which evaluations are based and the supporting data in the financial statements. Our independent auditors and our internal auditors are invited to attend all meetings of audit committee when it is acting in the role of the financial statements examination committee.
Compensation Committee
Amendment no. 20 to the Israeli Companies Law was published on November 12, 2012 and became effective on December 12, 2012, or Amendment no. 20. In general, Amendment no. 20 requires public companies to appoint a compensation committee and to adopt a compensation policy with respect to its officers, or the Compensation Policy. In addition, Amendment no. 20 addresses the corporate approval process required for a public company’s engagement with its officers (with specific reference to a director, a non-director officer, a chief executive officer and controlling shareholders and their relatives who are employed by the company).
The compensation committee shall be nominated by the board of directors and be comprised of its members. The compensation committee must consist of at least three members. All of the external directors must serve on the compensation committee and constitute a majority of its members. The remaining members of the compensation committee must be directors who qualify to serve as members of the audit committee (including the fact that they are independent) and their compensation should be identical to the compensation paid to the external directors of the company.
Similar to the rules that apply to the audit committee, the compensation committee may not include the chairman of the board, or any director employed by the Company, by a controlling shareholder or by any entity controlled by a controlling shareholder, or any director providing services to the company, to a controlling shareholder or to any entity controlled by a controlling shareholder on a regular basis, or any director whose primary income is dependent on a controlling shareholder, and may not include a controlling shareholder or any of its relatives. Individuals who are not permitted to be compensation committee members may not participate in the committee’s meetings other than to present a particular issue; provided, however, that an employee that is not a controlling shareholder or relative may participate in the committee’s discussions, but not in any vote, and our legal counsel and corporate secretary may participate in the committee’s discussions and votes if requested by the committee.
The roles of the compensation committee are, among others, to: (i) recommend to the board of directors the Compensation Policy for office holders and recommend to the board once every three years the extension of a Compensation Policy that had been approved for a period of more than three years; (ii) recommend to the directors any update of the Compensation Policy, from time to time, and examine its implementation; (iii) decide whether to approve the terms of office and of employment of office holders that require approval of the compensation committee; and (iv) decide, in certain circumstances, whether to exempt the approval of terms of office of a chief executive officer from the requirement of shareholder approval.
100
The Compensation Policy requires the approval of the general meeting of shareholders with a “Special Majority”, which requires a majority of the shareholders of the company who are not either a controlling shareholder or an “interested party” in the proposed resolution, or that shareholders holding less than 2% of the voting power in the company voted against the proposed resolution at such meeting. However, under special circumstances, the board of directors may approve the compensation policy without shareholder approval, if the compensation committee and thereafter the board of directors decided, based on substantiated reasons after they have reviewed the Compensation Policy again, that the Compensation Policy is in the best interest of the company. The Compensation Policy is required to be brought before the shareholders of the Company once every three years for approval.
Under the Israeli Companies Law, our Compensation Policy must generally serve as the basis for corporate approvals with respect to the financial terms of employment or engagement of office holders, including exemption, insurance, indemnification or any monetary payment or obligation of payment in respect of employment or engagement. The Compensation Policy must relate to certain factors, including advancement of the company’s objective, the company’s business plan and its long term strategy, and creation of appropriate incentives for office holders. It must also consider, among other things, the company’s risk management, size and nature of its operations. The Compensation Policy must furthermore consider the following additional factors:
| ● | The knowledge, skills, expertise, and accomplishments of the relevant office holder; |
| ● | The office holder’s roles and responsibilities and prior compensation agreements with him or her; |
| ● | The relationship between the terms offered and the average compensation of the other employees of the company, including those employed through manpower companies; |
| ● | The impact of disparities in salary upon work relationships in the company; |
| ● | The possibility of reducing variable compensation at the discretion of the board of directors; |
| ● | The possibility of setting a limit on the exercise value of non-cash variable equity-based compensation; and |
| ● | As to severance compensation, the period of service of the office holder, the terms of his or her compensation during such service period, the company’s performance during that period of service, the person’s contributions towards the company’s achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company. | |
| The Compensation Policy must also include the following principles: | ||
| ● | the link between variable compensation and the long term performance and measurable criteria; |
| ● | the relationship between variable and fixed compensation, and the ceiling for the value of variable compensation; |
| ● | the conditions under which an office holder would be required to repay compensation paid to him or her if it was later shown that the data upon which such compensation was based was inaccurate and was required to be restated in the company’s financial statements; |
| ● | the minimum holding or vesting period for variable, equity-based compensation; and |
| ● | maximum limits for severance compensation. |
The Compensation Policy was approved by the general meeting of shareholders on January 19, 2017 after discussions and recommendation of the compensation committee and approval by the Board of Directors. Moreover, the approval of the compensation committee is required in order to approve terms of office and/or employment of office holders.
101
Yaacov Goldman is the chairman of our compensation committee. Israel Shamay and Guy Regev serve as the other members of our compensation committee.
Under Amendment no. 27 to the Israeli Companies Law, which became effective as of February 17, 2016, the audit committee of an Israeli public company which has been established and conducts itself also in accordance with provisions governing the composition of the compensation committee as set forth in the Israeli Companies Law, may act in lieu of a compensation committee with respect to the responsibilities of a compensation committee which are set forth in the Israeli Companies Law.
Approval of Related Party Transactions under the Israeli Companies Law
Fiduciary duties of the office holders
The Israeli Companies Law imposes a duty of care and a duty of loyalty on all office holders of a company. The duty of care of an office holder is based on the duty of care set forth in connection with the tort of negligence under the Israeli Torts Ordinance (New Version) 5728-1968. This duty of care requires an office holder to act with the degree of proficiency with which a reasonable office holder in the same position would have acted under the same circumstances. The duty of care includes a duty to use reasonable means, in light of the circumstances, to obtain:
| ● | information on the advisability of a given action brought for his or her approval or performed by virtue of his or her position; and |
| ● | all other important information pertaining to these action. |
The duty of loyalty requires an office holder to act in good faith and for the benefit of the company, and includes the duty to:
| ● | refrain from any act involving a conflict of interest between the performance of his or her duties in the company and his or her other duties or personal affairs; |
| ● | refrain from any activity that is competitive with the business of the company; |
| ● | refrain from exploiting any business opportunity of the company for the purpose of gaining a personal advantage for himself or herself or others; and |
| ● | disclose to the company any information or documents relating to our affairs which the office holder received as a result of his or her position as an office holder. |
We may approve an act performed in breach of the duty of loyalty of an office holder provided that the office holder acted in good faith, the act or its approval does not harm the company, and the office holder discloses his or her personal interest, as described below.
Disclosure of personal interests of an office holder and approval of acts and transactions
The Israeli Companies Law requires that an office holder promptly disclose to the company any personal interest that he or she may have and all related material information or documents relating to any existing or proposed transaction by the company. An interested office holder’s disclosure must be made promptly and in any event no later than the first meeting of the board of directors at which the transaction is considered. An office holder is not obligated to disclose such information if the personal interest of the office holder derives solely from the personal interest of his or her relative in a transaction that is not considered as an extraordinary transaction.
The term personal interest is defined under the Israeli Companies Law to include the personal interest of a person in an action or in the business of a company, including the personal interest of such person’s relative or the interest of any corporation in which the person is an interested party, but excluding a personal interest stemming solely from the fact of holding shares in the company. A personal interest furthermore includes the personal interest of a person for whom the office holder holds a voting proxy or the interest of the office holder with respect to his or her vote on behalf of the shareholder for whom he or she holds a proxy even if such shareholder itself has no personal interest in the approval of the matter. An office holder is not, however, obliged to disclose a personal interest if it derives solely from the personal interest of his or her relative in a transaction that is not considered an extraordinary transaction.
102
Under the Israeli Companies Law, an extraordinary transaction which requires approval is defined as any of the following:
| ● | a transaction other than in the ordinary course of business; |
| ● | a transaction that is not on market terms; or |
| ● | a transaction that may have a material impact on our profitability, assets or liabilities. |
Under the Israeli Companies Law, once an office holder has complied with the disclosure requirement described above, a company may approve a transaction between the company and the office holder or a third party in which the office holder has a personal interest, or approve an action by the office holder that would otherwise be deemed a breach of duty of loyalty. However, a company may not approve a transaction or action that is adverse to our interest or that is not performed by the office holder in good faith.
Under the Israeli Companies Law, unless the articles of association of a company provide otherwise, a transaction with an office holder, a transaction with a third party in which the office holder has a personal interest, and an action of an office holder that would otherwise be deemed a breach of duty of loyalty requires approval by the board of directors. Our Amended and Restated Articles of Association do not provide otherwise. If the transaction or action considered is (i) an extraordinary transaction, (ii) an action of an office holder that would otherwise be deemed a breach of duty of loyalty and may have a material impact on a company’s profitability, assets or liabilities, (iii) an undertaking to indemnify or insure an office holder who is not a director, or (iv) for matters considered an undertaking concerning the terms of compensation of an office holder who is not a director, including, an undertaking to indemnify or insure such office holder, then approval by the audit committee is required prior to approval by the board of directors. Arrangements regarding the compensation, indemnification or insurance of a director require the approval of the audit committee, board of directors and shareholders, in that order.
A director who has a personal interest in a matter that is considered at a meeting of the board of directors or the audit committee may generally not be present at the meeting or vote on the matter, unless a majority of the directors or members of the audit committee have a personal interest in the matter or the chairman of the audit committee or board of directors, as applicable, determines that he or she should be present to present the transaction that is subject to approval. If a majority of the directors have a personal interest in the matter, such matter would also require approval of the shareholders of the company.
Disclosure of personal interests of a controlling shareholder and approval of transactions
Under the Israeli Companies Law and a recent amendment thereto, the disclosure requirements that apply to an office holder also apply to a controlling shareholder of a public company. See “— Audit Committee” for a definition of controlling shareholder. Extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, including a private placement in which a controlling shareholder has a personal interest, as well as transactions for the provision of services whether directly or indirectly by a controlling shareholder or his or her relative, or a company such controlling shareholder controls, and transactions concerning the terms of engagement of a controlling shareholder or a controlling shareholder’s relative, whether as an office holder or an employee, require the approval of the audit committee, the board of directors and a majority of the shares voted by the shareholders of the company participating and voting on the matter in a shareholders’ meeting. In addition, such shareholder approval must fulfill one of the following requirements:
| ● | at least a majority of the shares held by shareholders who have no personal interest in the transaction and are voting at the meeting must be voted in favor of approving the transaction, excluding abstentions; or | |
| ● | the shares voted by shareholders who have no personal interest in the transaction who vote against the transaction represent no more than 2% of the voting rights in the company. |
103
To the extent that any such transaction with a controlling shareholder is for a period extending beyond three years, approval is required once every three years, unless the audit committee determines that the duration of the transaction is reasonable given the circumstances related thereto.
Duties of shareholders
Under the Israeli Companies Law, a shareholder has a duty to refrain from abusing its power in the company and to act in good faith and in an acceptable manner in exercising its rights and performing its obligations to the company and other shareholders, including, among other things, voting at general meetings of shareholders on the following matters:
| ● | an amendment to the articles of association; | |
| ● | an increase in our authorized share capital; |
| ● | a merger; |
| ● | an increase in our authorized share capital; and |
| ● | the approval of related party transactions and acts of office holders that require shareholder approval. |
A shareholder also has a general duty to refrain from discriminating against other shareholders.
The remedies generally available upon a breach of contract will also apply to a breach of the above mentioned duties, and in the event of discrimination against other shareholders, additional remedies are available to the injured shareholder.
In addition, any controlling shareholder, any shareholder that knows that its vote can determine the outcome of a shareholder vote and any shareholder that, under a company’s articles of association, has the power to appoint or prevent the appointment of an office holder, or has another power with respect to a company, is under a duty to act with fairness towards the company. The Israeli Companies Law does not describe the substance of this duty except to state that the remedies generally available upon a breach of contract will also apply in the event of a breach of the duty to act with fairness, taking the shareholder’s position in the company into account.
Exculpation, Insurance and Indemnification of Directors and Officers
Under the Israeli Companies Law, a company may not exculpate an office holder from liability for a breach of the duty of loyalty. An Israeli company may exculpate an office holder in advance from liability to us, in whole or in part, for damages caused to us as a result of a breach of duty of care but only if a provision authorizing such exculpation is included in its articles of association. Our Amended and Restated Articles of Association include such a provision. We may not exculpate in advance a director from liability arising out of a prohibited dividend or distribution to shareholders.
Under the Israeli Companies Law and the Israeli Securities Law, a company may indemnify, or undertake in advance to indemnify, an office holder, provided its articles of association include a provision authorizing such indemnification, for the following liabilities and expenses imposed on an office holder or incurred by office holder due to acts performed by him or her as an office holder:
| ● | Financial liability incurred by or imposed on him or her in favor of another person pursuant to a judgment, including a settlement or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on our activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned foreseen events and amount or criteria; |
104
| ● | Reasonable litigation expenses, including attorneys’ fees, incurred by the office holder as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no indictment was filed against such office holder as a result of such investigation or proceeding; and (ii) no financial liability was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent or as a monetary sanction; |
| ● | Reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by us, on our behalf, or by a third party, or in connection with criminal proceedings in which the office holder was acquitted, or as a result of a conviction for an offense that does not require proof of criminal intent; and |
| ● | Expenses, including reasonable litigation expenses and legal fees, incurred by an office holder in relation to an administrative proceeding instituted against such office holder, or certain compensation payments required to be made to an injured party, pursuant to certain provisions of the Israeli Securities Law. |
Under the Israeli Companies Law, a company may insure an office holder against the following liabilities incurred for acts performed by him or her as an office holder if and to the extent provided in the Company’s articles of association:
| ● | a breach of the duty of loyalty to us, provided that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm us; |
| ● | a breach of duty of care to us or to a third party; and |
| ● | a financial liability imposed on the office holder in favor of a third party. |
Subject to the provisions of the Israeli Companies Law and the Israeli Securities Law, we may also enter into a contract to insure an office holder, in respect of expenses, including reasonable litigation expenses and legal fees, incurred by an office holder in relation to an administrative proceeding instituted against such office holder or payment required to be made to an injured party, pursuant to certain provisions of the Israeli Securities Law.
Nevertheless, under the Israeli Companies Law, a company may not indemnify, exculpate or insure an office holder against any of the following:
| ● | a breach of fiduciary duty, except for indemnification and insurance for a breach of the duty of loyalty to us in the event office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice us; |
| ● | a breach of duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder; |
| ● | an act or omission committed with intent to derive unlawful personal benefit; or |
| ● | a fine, monetary sanction, penalty or forfeit levied against the office holder. |
Under the Israeli Companies Law, exculpation, indemnification and insurance of office holders require the approval of the compensation committee, board of directors and, in certain circumstances, the shareholders. Our Amended and Restated Articles of Association permit us to exculpate, indemnify and insure our office holders to the fullest extent permitted by the Israeli Companies Law.
105
Approval of Compensation to Our Officers
The Israeli Companies Law prescribes that compensation to officers must be approved by a company’s Board of Directors after obtaining the approval of the compensation committee.
As detailed above, our compensation committee consists of three independent directors: Israel Shamay, Yaacov Goldman and Guy Regev. The responsibilities of the compensation committee are to set our overall policy on executive remuneration and to decide the specific remuneration, benefits and terms of employment for directors, officers and the Chief Executive Officer.
The objectives of the compensation committee’s policies are that such individuals should receive compensation which is appropriate given their performance, level of responsibility and experience. Compensation packages should also allow us to attract and retain executives of the necessary caliber while, at the same time, motivating them to achieve the highest level of corporate performance in line with the best interests of shareholders. In order to determine the elements and level of remuneration appropriate to each executive director, the compensation committee reviews surveys on executive pay, obtains external professional advice and considers individual performance.
Internal Auditor
Under the Israeli Companies Law, the board of directors must appoint an internal auditor, nominated by the audit committee. The role of the internal auditor is to examine, among other matters, whether our actions comply with the law and orderly business procedure. Under the Israeli Companies Law, an internal auditor may not be:
| ● | a person (or a relative of a person) who holds more than 5% of our ordinary shares; |
| ● | a person (or a relative of a person) who has the power to appoint a director or the general manager of the company; |
| ● | an executive officer or director of the company (or a relative thereof); or |
| ● | a member of our independent accounting firm, or anyone on his or her behalf. |
We comply with the requirement of the Israeli Companies Law relating to internal auditors. Our internal auditors examine whether our various activities comply with the law and orderly business procedure. Our current internal auditor is Deloitte.
D. Employees.
As of December 31, 2018, we had seven employees, three of whom were employed in management and administration, three of whom were employed in research and development and one of whom was employed in business development. All of these employees were located in Israel.
While none of our employees are party to any collective bargaining agreements, certain provisions of the collective bargaining agreements between the Histadrut (General Federation of Labor in Israel) and the Coordination Bureau of Economic Organizations (including the Industrialists’ Associations) are applicable to our employees by order of the Israel Ministry of Labor. These provisions primarily concern the length of the workday, minimum daily wages for professional workers, pension fund benefits for all employees, insurance for work-related accidents, procedures for dismissing employees, determination of severance pay and other conditions of employment. We generally provide our employees with benefits and working conditions beyond the required minimums. We have never experienced any employment-related work stoppages and believe our relationship with our employees is good.
106
E. Share Ownership.
The following table sets forth information regarding the beneficial ownership of our outstanding ordinary shares as of March 21, 2019 by the members of our senior management and board of directors individually and as a group. The beneficial ownership of ordinary shares is based on the 44,875,482 ordinary shares outstanding as of March 21, 2019 and is determined in accordance with the rules of the SEC and generally includes any ordinary shares over which a person exercises sole or shared voting or investment power. For purposes of the table below, we deem shares subject to options or warrants that are currently exercisable or exercisable within 60 days of March 21, 2019, to be outstanding and to be beneficially owned by the person holding the options or warrants for the purposes of computing the percentage ownership of that person but we do not treat them as outstanding for the purpose of computing the percentage ownership of any other person.
| Name of Beneficial Owner | Number of Ordinary Shares | Percentage of Class* | ||||||
| Senior Management and Directors | ||||||||
| Ilan Cohn, Ph.D. | 151,567 | (1) | * | |||||
| Pnina Fishman, Ph.D. | 595,633 | (2) | 1.3 | % | ||||
| Motti Farbstein | 155,507 | (3) | * | |||||
| Sari Fishman, Ph.D. | 144,875 | (4) | * | |||||
| Guy Regev | 52,240 | (5) | * | |||||
| Abraham Sartani, M.D. | 22,000 | (6) | * | |||||
| Israel Shamay | 18,000 | (7) | * | |||||
| Yaacov Goldman | 18,000 | (7) | * | |||||
| Senior Management and Directors as a group (8 persons) | 1,157,822 | 2.8 | % | |||||
| Holders of more than 5% of our voting securities | ||||||||
| Meitav Dash Investments Ltd. | 2,990,321 | (8) | 6.7 | % | ||||
| Anson Investments Master Fund LP | 4,476,192 | (9) | 9.9 | % | ||||
| * | Denotes less than 1% |
| (1) | Represents (i) 133,567 ordinary shares, and (ii) 18,000 options to purchase 18,000 ordinary shares at an exercise price of NIS 2.926 per option and expire on November 8, 2027. Excludes 30,000 options to purchase 30,000 ordinary shares that vest in more than 60 days from March 21, 2019. |
| (2) | Represents (i) 263,433 ordinary shares, (ii) 2,680,000 options to purchase 107,200 ordinary shares at an exercise price of NIS 0.644 per option and expiring on January 13, 2021, (iii) 200,000 options to purchase 200,000 ordinary shares at an exercise price of NIS 3.573 per option and expire on October 22, 2025 and (iv) 25,000 options to purchase 25,000 ordinary shares at an exercise price of NIS 2.344 per option and expire on January 7, 2029. Excludes 375,000 options to purchase 375,000 ordinary shares that vest in more than 60 days from March 21, 2019. |
| (3) | Represents (i) 1,257 ordinary shares, (ii) 200,000 options to purchase 8,000 ordinary shares, of which (1) 100,000 are exercisable into 4,000 ordinary shares at an exercise price of NIS 0.385 per option and expire on May 2, 2022, and (2) 100,000 are exercisable into 4,000 ordinary shares at an exercise price of NIS 0.326 per option and expire on March 20, 2023, (iii) 10,000 options to purchase 10,000 ordinary shares at an exercise price of NIS 8.1205 per option and expire on March 18, 2025, (iv) 48,750 options to purchase 48,750 ordinary shares at an exercise price of NIS 4.317 per option and expire on February 18, 2026, (v) 78,125 options to purchase 78,125 ordinary shares at an exercise price of NIS 2.513 per option and expire on December 28, 2027 and (vi) 9,375 options to purchase 9,375 ordinary shares at an exercise price of NIS 2.344 per option and expire on January 7, 2029. Excludes 323,750 options to purchase 323,750 ordinary shares that vest in more than 60 days from March 21, 2019. |
| (4) | Represents (i) 200,000 options to purchase 8,000 ordinary shares, of which (1) 100,000 are exercisable into 4,000 ordinary shares at an exercise price of NIS 0.385 per option and expire on May 2, 2022, and (2) 100,000 are exercisable into 4,000 ordinary shares at an exercise price of NIS 0.326 per option and expire on March 20, 2023, and (ii) 10,000 options to purchase 10,000 ordinary shares at an exercise price of NIS 8.1205 per option and expire on March 18, 2025, (iii) 32,500 options to purchase 32,500 ordinary shares at an exercise price of NIS 4.317 per option and expire on February 18, 2026, (iv) 40,000 options to purchase 40,000 ordinary shares at an exercise price of NIS 3.662 per option and expire on March 30, 2027, (v) 46,875 options to purchase 46,875 ordinary shares at an exercise price of NIS 2.513 per option and expire on December 30, 2027, and (vi) 7,500 options to purchase 7,500 ordinary shares at an exercise price of NIS 2.344 per option and expire on January 7, 2029. Excludes 263,125 options to purchase 263,125 ordinary shares that vest in more than 60 days from March 21, 2019. |
107
| (5) | Represents (i) 24,240 ordinary shares, (ii) 250,000 options are exercisable into 10,000 ordinary shares at an exercise price of NIS 0.60 per option and expire on May 2, 2023, (iii) 18,000 options are exercisable into 18,000 ordinary shares at an exercise price of NIS 2.926 per option and expire on December 28, 2027. Excludes 30,000 options to purchase 30,000 ordinary shares that vest in more than 60 days from March 21, 2019. |
| (6) | Represents (i) 100,000 options to purchase 4,000 ordinary shares at an exercise price of NIS 0.60 per option and expire on August 14, 2022, and (ii) 18,000 options to purchase 18,000 ordinary shares at an exercise price of NIS 2.926 per option and expire on November 8, 2027. Excludes 30,000 options to purchase 30,000 ordinary shares that vest in more than 60 days from March 21, 2019. |
| (7) | Represents 18,000 options to purchase 18,000 ordinary shares at an exercise price of NIS 2.926 per option and expire on November 8, 2027. Excludes 30,000 options to purchase 30,000 ordinary shares that vest in more than 60 days from March 21, 2019. |
| (8) | Based on information contained in a Schedule 13G/A filed with the SEC on March 19, 2019 jointly by Meitav Dash Investments Ltd. (“Meitav Investments”) and Meitav Dash Provident Funds Ltd. and Tachlit Indices Mutual Fund Management Ltd. (“Meitav Funds”) The ordinary shares are beneficially owned by various direct or indirect, majority or wholly-owned subsidiaries of Meitav Investments (the “Subsidiaries”). Meitav Investments, Meitav Funds and the Subsidiaries disclaim any beneficial ownership of the ordinary shares referred to herein in excess of their actual pecuniary interest therein and each of Meitav Investments, Meitav Funds and the Subsidiaries disclaim beneficial ownership of any such ordinary shares. |
| (9) | Based on information contained in a Schedule 13G filed with the SEC on February 4, 2019 jointly by Anson Funds Management LP (d/b/a Anson Group), or AFM, Anson Management GP LLC, or AM, Bruce R. Winson, the principal of AFM and AM, Anson Advisors Inc. (d/b/a Anson Funds), or AA, Amin Nathoo, a director of AA, and Moez Kassam, a director of AA. AMF and AA serve as co-investment advisors to a private fund, or the Fund, and may direct the vote and disposition of the 4,476,192 ordinary shares represented by ADSs held by the Fund. As the general partner of AFM, AM may direct the vote and disposition of the 4,476,192 ordinary shares represented by ADSs held by the Fund. As the principal of AFM and AM, Mr. Winson may direct the vote and disposition of the 4,476,192 ordinary shares represented by ADSs held by the Fund. As directors of AA, Mr. Nathoo and Mr. Kassam may each direct the vote and disposition of the 4,476,192 ordinary shares represented by ADSs held by the Fund. |
To our knowledge the significant changes in the percentage of ownership held by our major shareholders reported in our Annual Reports on Form 20-F during the past three have been (i) the decrease in 2016 below 5%, the increase above 5% in 2018 and the later decrease below 5% in the percentage ownership held by Sabby Management, LLC, (ii) the increase in 2018 above 5%, the later decrease below 5% and the increase above 5% in 2019 in the percentage ownership held by Anson, and (iii) the increase in 2019 above 5% in the percentage ownership held by Meitav Dash.
The Bank of New York Mellon, or BNY, is the holder of record for our ADR program, pursuant to which each ADS represents 2 ordinary shares. As of March 21, 2019, BNY held 29,554,441 ordinary shares representing approximately 66% of the outstanding ordinary shares at that date. Certain of these ordinary shares were held by brokers or other nominees. As a result, the number of holders of record or registered holders in the United States is not representative of the number of beneficial holders or of the residence of beneficial holders.
108
Share Option Plans
We maintain the following share option plans for our and our subsidiary’s employees, directors and consultants. In addition to the discussion below, see Note 12b of our consolidated financial statements, included elsewhere in this Annual Report on Form 20-F.
Our Board of Directors administers our share option plans and has the authority to designate all terms of the options granted under our plans including the grantees, exercise prices, grant dates, vesting schedules and expiration dates, which may be no more than ten years after the grant date. Options may not be granted with an exercise price of less than the fair market value of our ordinary shares on the date of grant, unless otherwise determined by our Board of Directors.
As of December 31, 2018, options to purchase an aggregate of 2,177,400 ordinary shares, par value NIS 0.25, are outstanding pursuant to the 2003 and 2013 share option plans.
2003 Share Option Plan
Under the 2003 Plan we granted options during the period between 2003 and 2013, at exercise prices between NIS 0.25 and NIS 31.175 per ordinary share, par value NIS 0.25. Options to purchase up to 1,132,514 ordinary shares, par value NIS 0.25, were available to be granted under the 2003 Plan. As of December 31, 2018, 3,810,000 options to purchase 125,400 ordinary shares were outstanding. Options granted to Israeli employees were in accordance with section 102 of the Income Tax Ordinance, 1961, or the Tax Ordinance, under the capital gains option set forth in section 102(b)(2) of the Tax Ordinance. The options are non-transferable.
The option term is for a period of ten years from the grant date. The options were granted for no consideration. The options vest over a four or two year period. As of March 21, 2019, 152,400 options to purchase 152,400 ordinary shares, par value NIS 0.25, were fully vested.
2013 Share Option Plan
Under the 2013 Plan we granted options at exercise prices between NIS 3.573 and NIS 12 per ordinary share, par value NIS 0.25. Options to purchase up to 2,500,000 ordinary shares, par value NIS 0.25, were available to be granted under the 2013 Plan. As of December 31, 2018, 2,025,000 options to purchase 2,025,000 ordinary shares were outstanding. Options granted to Israeli employees were in accordance with the Tax Ordinance under the capital gains option set forth in section 102(b)(2) of the Tax Ordinance. The options are non-transferable.
The option term is for a period of ten years from the grant date. The options were granted for no consideration. The options vest over a four year period. As of March 21, 2019, options to purchase 729,129 ordinary shares, par value NIS 0.25, were fully vested.
ITEM 7. Major Shareholders and Related Party Transactions
A. Major Shareholders.
Except as set forth in “Item 6. Directors, Senior Management and Employees—E. Share Ownership,” to the best of our knowledge, no other person who we know beneficially owns 5.0% or more of the Company’s ordinary shares outstanding as of March 21, 2019. None of our shareholders has different voting rights from other shareholders. Other than as described herein, to the best of our knowledge, we are not owned or controlled, directly or indirectly, by another corporation, by any foreign government or by any natural person or legal persons, severally or jointly, and we are not aware of any arrangement that may, at a subsequent date, result in a change of control of our Company.
B. Related Party Transactions.
The following is a description of the transactions with related parties to which we, or our subsidiaries, are party, and which were in effect since January 1, 2016. The descriptions provided below are summaries of the terms of such agreements, do not purport to be complete and are qualified in their entirety by the complete agreements.
109
We believe that we have executed all of our transactions with related parties on terms no less favorable to us than those we could have obtained from unaffiliated third parties. We are required by Israeli law to ensure that all future transactions between us and our officers, directors and principal shareholders and their affiliates are approved by a majority of our Board of Directors, including a majority of the independent and disinterested members of our Board of Directors, and that they are on terms no less favorable to us than those that we could obtain from unaffiliated third parties.
OphthaliX Merger Agreement
On May 21, 2017, our now former subsidiary, OphthaliX and a wholly-owned private Israeli subsidiary of OphthaliX, Bufiduck Ltd., or Merger Sub, and Wize Pharma Ltd., an Israeli company formerly listed on the Tel Aviv Stock Exchange, or Wize Israel, entered into an Agreement and Plan of Merger, or the Merger Agreement. On October 31, 2017, OphthaliX entered into an amendment to the Merger Agreement with Merger Sub and Wize Israel extending the Expiration Date (as defined in the Merger Agreement) to November 30, 2017.
Concurrently with the execution of the Merger Agreement and as contemplated therein, we entered into a Voting and Undertaking Agreement with OphthaliX and Wize Israel, or the Voting Agreement, pursuant to which we agreed to vote our shares of OphthaliX held by us in favor of approving the matters on the agenda of the annual general meeting and against any actions that could adversely affect the consummation of the Merger. In addition, the Voting Agreement placed certain restrictions on the transfer of the shares of OphthaliX held by us and we agreed to indemnify Wize Israel and OphthaliX with respect to certain liabilities of OphthaliX occurring in the period up to the closing of the Merger but excluding certain liabilities in respect of any legal proceedings arising out of or related to the transactions contemplated by the Merger Agreement.
On November 16, 2017, OphthaliX (which has been renamed “Wize Pharma, Inc.”) completed its transaction with Wize Israel in accordance with the terms of the Merger Agreement pursuant to which Merger Sub merged with and into Wize Israel, with Wize Israel surviving as a wholly owned subsidiary of the Company, or the Merger. In connection with the Merger and under the terms of the Merger Agreement, at the effective time of the Merger, each ordinary share of Wize Israel that was issued and outstanding was automatically cancelled and converted into 4.1445791236989 shares of common stock of OphthaliX. As a result, an aggregate of 93,971,259 shares of common stock of OphthaliX were issued to former Wize Israel shareholders. The pre-Merger stockholders of OphthaliX retained an aggregate of 10,441,251 shares of the common stock of OphthaliX. Consequently, our ownership of OphthaliX, which consisted of 8,563,254 shares of common stock, was reduced from approximately 82% immediately prior to the Merger to approximately 8% immediately after the Merger.
Immediately prior to the effective time of the Merger, OphthaliX sold on an “as is” basis to us all the ordinary shares of Eye-Fite in exchange for the irrevocable cancellation and waiver of all indebtedness owed by the OphthaliX and Eye-Fite to us, including approximately $5 million of deferred payments owed by OphthaliX and Eye-Fite to us and, as part of the purchase of Eye-Fite, we also assumed certain accrued milestone payments in the amount of $175,000 under a license agreement previously entered into with NIH. In addition, that certain exclusive license between us and OphthaliX and a related services agreement were terminated pursuant to a Termination of License Agreement and a Termination of Services Agreement that was entered into in connection with the closing of the Merger. Immediately following the Merger, OphthaliX continued to hold 446,827 of our ordinary shares.
The foregoing share amounts of OphthaliX do not give effect to a 1-for-24 reverse stock split of OphthaliX that took effect, subsequent to the completion of the Merger, on March 5, 2018.
Employment and Consulting Agreements
We have or have had employment, consulting or related agreements with each member of our senior management. See “Item 6. Directors, Senior Management and Employees—Compensation”.
We employ Zivit Harpaz as Director of Regulatory and Clinical Operations. For fiscal years 2018, 2017 and 2016, Ms. Harpaz received salary, bonus and benefits totaling approximately NIS 589,000, NIS 532,000, and NIS 522,000, respectively, and during fiscal years 2019, 2017 and 2016 we awarded to Ms. Harpaz options to purchase 70,000, 125,000, and 30,000 ordinary shares, respectively. Ms. Harpaz is the daughter of Pnina Fishman.
110
Options
We have granted options to purchase our ordinary shares to certain of our senior management and directors. See “Item 6. B.—Compensation” and “Item 6. Directors, Senior Management and Employees—Share Ownership”. We describe our option plans under “Item 6. Directors, Senior Management and Employees—Share Ownership”.
Indemnification Agreements
Our Amended and Restated Articles of Association permit us to exculpate, indemnify and insure our directors and officeholders to the fullest extent permitted by the Israeli Companies Law. We have obtained directors’ and officers’ insurance for each of our officers and directors and have entered into indemnification agreements with all of our current officers and directors.
C. Interests of Experts and Counsel.
Not applicable.
A. Consolidated Financial Statements and Other Financial Information
See “Item 18. Financial Statements” for a list of all financial statements filed as part of this Annual Report on Form 20-F.
Legal Matters
We are not involved in any legal or arbitration proceedings that may have or have had in the recent past, significant effects on our financial position or profitability.
On June 29, 2015, we received a lawsuit, filed with the District Court of Tel-Aviv, requesting recognition of this lawsuit as a class action. The lawsuit named the Company, its Chief Executive Officer and directors as defendants. The lawsuit alleges, among other things, that we misled the public with regard to disclosures concerning the efficacy of our drug candidate, Piclidenoson. The claimant alleges that he suffered personal damages of over NIS 73,000, while also claiming that our shareholders suffered damages of approximately NIS 125 million. On March 31, 2016, we filed a response to the lawsuit. On March 1, 2017, a hearing was held in the District Court on whether to certify the lawsuit as a class action. A final hearing on the certification was held on May 17, 2017. On July 18, 2017, the District Court of Tel-Aviv issued a ruling in which it denied the request to recognize the lawsuit as a class action and awarded us an amount of NIS 50,000 to pay our expenses in relation to such law suit. The claimant filed a petition with the Supreme Court appealing the District Court decision. On January 28, 2018, the Supreme Court issued a notice of procedures to be complied with by the relevant parties leading up to a formal hearing scheduled for December 5, 2018. On December 5, 2018, the Supreme Court dismissed the appeal and as part of a compromise by the claimant not to pursue the appeal, the Supreme Court ordered us to return the aforementioned expenses to the claimant. Accordingly, this lawsuit has been finally dismissed and is no longer pending against us.
Dividend Policy
We have never declared or paid cash dividends to our shareholders. Currently we do not intend to pay cash dividends. We intend to reinvest any earnings in developing and expanding our business. Any future determination relating to our dividend policy will be at the discretion of our Board of Directors and will depend on a number of factors, including future earnings, our financial condition, operating results, contractual restrictions, capital requirements, business prospects, applicable Israeli Companies Law and other factors our Board of Directors may deem relevant.
111
B. Significant Changes
See “Note 19:- Subsequent Events” to our consolidated financial statements included in this Annual Report beginning on page F-1 for a discussion of significant events that have occurred since December 31, 2018.
A. Offer and Listing Details
Ordinary Shares
Our ordinary shares have been trading on the TASE under the symbol “CFBI” since October 2005.
ADSs
On October 2, 2012, our ADSs began trading OTC in the United States under the symbol “CANFY” and on November 19, 2013, our ADSs began trading on the NYSE American under the symbol “CANF.” One ADS represents two ordinary shares. See “Item 12. Description of Securities Other Than Equity Securities—D. American Depositary Shares” for a description of the rights attaching to our ADSs. See “Item 12. Description of Securities Other Than Equity Securities—D. American Depositary Shares” for a description of the rights attaching to our ADSs.
B. Plan of Distribution.
Not applicable.
C. Markets.
See “—Offer and Listing Details” above.
D. Selling Shareholders.
Not applicable.
E. Dilution.
Not applicable.
F. Expenses of the Issue.
Not applicable.
ITEM 10. Additional Information
A. Share Capital.
Not applicable.
B. Memorandum and Articles of Association.
Our number with the Israeli Registrar of Companies is 512022153. Our purpose is set forth in Section 3 of our Articles of Association and includes every lawful purpose.
Our ordinary shares that are fully paid for are issued in registered form and may be freely transferred under our Amended and Restated Articles of Association, unless the transfer is restricted or prohibited by applicable law or the rules of a stock exchange on which the shares are traded. The ownership or voting of our ordinary shares by non-residents of Israel is not restricted in any way by our Amended and Restated Articles of Association or the laws of the State of Israel, except for ownership by nationals of some countries that are, or have been, in a state of war with Israel.
112
Pursuant to the Israeli Companies Law and our Amended and Restated Articles of Association, our board of directors may exercise all powers and take all actions that are not required under law or under our Amended and Restated Articles of Association to be exercised or taken by our shareholders, including the power to borrow money for company purposes.
Our Amended and Restated Articles of Association enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the Israeli Companies Law and must be approved by a resolution duly passed by our shareholders at a general or special meeting by voting on such change in the capital. In addition, transactions that have the effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings and profits and an issuance of shares for less than their nominal value, require a resolution of our board of directors and court approval.
Dividends
We may declare a dividend to be paid to the holders of our ordinary shares in proportion to their respective shareholdings. Under the Israeli Companies Law, dividend distributions are determined by the board of directors and do not require the approval of the shareholders of a company unless such company’s articles of association provide otherwise. Our Amended and Restated Articles of Association do not require shareholder approval of a dividend distribution and provide that dividend distributions may be determined by our board of directors.
Pursuant to the Israeli Companies Law, we may only distribute dividends from our profits accrued over the previous two years, as defined in the Israeli Companies Law, according to our then last reviewed or audited financial reports, or we may distribute dividends with court approval. In each case, we are only permitted to pay a dividend if there is no reasonable concern that payment of the dividend will prevent us from satisfying our existing and foreseeable obligations as they become due.
Election of Directors
Our ordinary shares do not have cumulative voting rights in the election of directors. As a result, the holders of a majority of the voting power represented at a shareholders meeting have the power to elect all of our directors, subject to the special approval requirements for external directors described under “Item 6. Directors, Senior Management and Employees—C. Board Practices — External Directors”.
Pursuant to our Amended and Restated Articles of Association, other than the external directors, for whom special election requirements apply under the Israeli Companies Law, our directors are elected at a general or special meeting of our shareholders and serve on the board of directors until the end of the next general meeting or they are removed by the majority of our shareholders at a general or special meeting of our shareholders or upon the occurrence of certain events, in accordance with the Israeli Companies Law and our Amended and Restated Articles of Association. In addition, our Amended and Restated Articles of Association allow our board of directors to appoint directors to fill vacancies on the board of directors to serve until the next general meeting or special meeting, or earlier if required by our Amended and Restated Articles of Association or applicable law. We have held elections for each of our non-external directors at each annual meeting of our shareholders since our initial public offering in Israel. External directors are elected for an initial term of three years and may be removed from office pursuant to the terms of the Israeli Companies Law.
See “Item 6. Directors, Senior Management and Employees—C. Board Practices—External Directors.”
Shareholder Meetings
Under Israeli Companies Law, we are required to hold an annual general meeting of our shareholders once every calendar year that must be no later than 15 months after the date of the previous annual general meeting. All meetings other than the annual general meeting of shareholders are referred to as special meetings. Our board of directors may call special meetings whenever it sees fit, at such time and place, within or outside of Israel, as it may determine. In addition, the Israeli Companies Law and our Amended and Restated Articles of Association provide that our board of directors is required to convene a special meeting upon the written request of (i) any two of our directors or one quarter of our board of directors or (ii) one or more shareholders holding, in the aggregate, either (1) 5% of our outstanding shares and 1% of our outstanding voting power or (2) 5% of our outstanding voting power.
113
Subject to the provisions of the Israeli Companies Law and the regulations promulgated thereunder, shareholders entitled to participate and vote at general meetings are the shareholders of record on a date to be decided by the board of directors, which may be between four and forty days prior to the date of the meeting. Furthermore, the Israeli Companies Law and our Amended and Restated Articles of Association require that resolutions regarding the following matters must be passed at a general meeting of our shareholders:
| ● | amendments to our Amended and Restated Articles of Association; |
| ● | appointment or termination of our auditors; |
| ● | appointment of directors and appointment and dismissal of external directors; |
| ● | approval of acts and transactions requiring general meeting approval pursuant to the Israeli Companies Law; |
| ● | director compensation, indemnification and change of the principal executive officer; |
| ● | increases or reductions of our authorized share capital; |
| ● | a merger; and |
| ● | the exercise of our Board of Director’s powers by a general meeting, if our board of directors is unable to exercise its powers and the exercise of any of its powers is required for our proper management. |
The Israeli Companies Law requires that a notice of any annual or special shareholders meeting be provided at least 21 days prior to the meeting and if the agenda of the meeting includes the appointment or removal of directors, the approval of transactions with office holders or interested or related parties, or an approval of a merger, notice must be provided at least 35 days prior to the meeting.
The Israeli Companies Law does not allow shareholders of publicly traded companies to approve corporate matters by written consent. Consequently, our Amended and Restated Articles of Association does not allow shareholders to approve corporate matters by written consent.
Pursuant to our Amended and Restated Articles of Association, holders of our ordinary shares have one vote for each ordinary share held on all matters submitted to a vote before the shareholders at a general meeting.
Quorum
The quorum required for our general meetings of shareholders consists of at least two shareholders present in person, by proxy or written ballot who hold or represent between them at least 25% of the total outstanding voting rights.
A meeting adjourned for lack of a quorum is adjourned to the same day in the following week at the same time and place or on a later date if so specified in the summons or notice of the meeting. At the reconvened meeting, any number of our shareholders present in person or by proxy shall constitute a lawful quorum.
Resolutions
Our Amended and Restated Articles of Association provide that all resolutions of our shareholders require a simple majority vote, unless otherwise required by applicable law.
114
Israeli law provides that a shareholder of a public company may vote in a meeting and in a class meeting by means of a written ballot in which the shareholder indicates how he or she votes on resolutions relating to the following matters:
| ● | an appointment or removal of directors; |
| ● | an approval of transactions with office holders or interested or related parties; |
| ● | an approval of a merger or any other matter in respect of which there is a provision in the articles of association providing that decisions of the general meeting may also be passed by written ballot; |
| ● | authorizing the chairman of the board of directors or his relative to act as our chief executive officer or act with such authority; or authorize our chief executive officer or his relative to act as the chairman of the board of directors or act with such authority; and |
| ● | other matters which may be prescribed by Israel’s Minister of Justice. |
The provision allowing the vote by written ballot does not apply where the voting power of the controlling shareholder is sufficient to determine the vote. Our Amended and Restated Articles of Association provide that our board of directors may prevent voting by means of a written ballot and this determination is required to be stated in the notice convening the general meeting.
The Israeli Companies Law provides that a shareholder, in exercising his or her rights and performing his or her obligations toward the company and its other shareholders, must act in good faith and in a customary manner, and avoid abusing his or her power. This is required when voting at general meetings on matters such as changes to the articles of association, increasing our registered capital, mergers and approval of related party transactions. A shareholder also has a general duty to refrain from depriving any other shareholder of its rights as a shareholder. In addition, any controlling shareholder, any shareholder who knows that its vote can determine the outcome of a shareholder vote and any shareholder who, under such company’s articles of association, can appoint or prevent the appointment of an office holder, is required to act with fairness towards the company. The Israeli Companies Law does not describe the substance of this duty except to state that the remedies generally available upon a breach of contract will also apply to a breach of the duty to act with fairness, and, to the best of our knowledge, there is no binding case law that addresses this subject directly.
Under the Israeli Companies Law, unless provided otherwise in a company’s articles of association, a resolution at a shareholders meeting requires approval by a simple majority of the voting rights represented at the meeting, in person, by proxy or written ballot, and voting on the resolution. A resolution for the voluntary winding up of the company requires the approval of holders of 75% of the voting rights represented at the meeting, in person, by proxy or by written ballot and voting on the resolution.
In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of our ordinary shares in proportion to their shareholdings. This right, as well as the right to receive dividends, may be affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future.
Access to Corporate Records
Under the Israeli Companies Law, all shareholders of a company generally have the right to review minutes of our general meetings, its shareholders register and principal shareholders register, articles of association, financial statements and any document it is required by law to file publicly with the Israeli Companies Registrar and the Israel Securities Authority. Any of our shareholders may request access to review any document in our possession that relates to any action or transaction with a related party, interested party or office holder that requires shareholder approval under the Israeli Companies Law. We may deny a request to review a document if we determine that the request was not made in good faith, that the document contains a commercial secret or a patent or that the document’s disclosure may otherwise prejudice our interests.
115
Acquisitions under Israeli Law
Full Tender Offer
A person wishing to acquire shares of a public Israeli company and who would as a result hold over 90% of the target company’s issued and outstanding share capital is required by the Israeli Companies Law to make a tender offer to all of our shareholders for the purchase of all of the issued and outstanding shares of the company. A person wishing to acquire shares of a public Israeli company and who would as a result hold over 90% of the issued and outstanding share capital of a certain class of shares is required to make a tender offer to all of the shareholders who hold shares of the same class for the purchase of all of the issued and outstanding shares of the same class. If the shareholders who do not accept the offer hold less than 5% of the issued and outstanding share capital of the company or of the applicable class, all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law (provided that a majority of the offerees that do not have a personal interest in such tender offer shall have approved the tender offer except that if the total votes to reject the tender offer represent less than 2% of the company’s issued and outstanding share capital, in the aggregate, approval by a majority of the offerees that do not have a personal interest in such tender offer is not required to complete the tender offer). However, a shareholder that had its shares so transferred may petition the court within six months from the date of acceptance of the full tender offer, whether or not such shareholder agreed to the tender or not, to determine whether the tender offer was for less than fair value and whether the fair value should be paid as determined by the court unless the acquirer stipulated in the tender offer that a shareholder that accepts the offer may not seek appraisal rights. If the shareholders who did not accept the tender offer hold 5% or more of the issued and outstanding share capital of the company or of the applicable class, the acquirer may not acquire shares of the company that will increase its holdings to more than 90% of our issued and outstanding share capital or of the applicable class from shareholders who accepted the tender offer.
Special Tender Offer
The Israeli Companies Law provides that an acquisition of shares of a public Israeli company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of 25% or more of the voting rights in the company, unless one of the exemptions in the Israeli Companies Law is met. This rule does not apply if there is already another holder of at least 25% of the voting rights in the company. Similarly, the Israeli Companies Law provides that an acquisition of shares in a public company must be made by means of a tender offer if as a result of the acquisition the purchaser would become a holder of 45% or more of the voting rights in the company, if there is no other shareholder of the company who holds 45% or more of the voting rights in the company, unless one of the exemptions in the Israeli Companies Law is met.
A special tender offer must be extended to all shareholders of a company but the offeror is not required to purchase shares representing more than 5% of the voting power attached to our outstanding shares, regardless of how many shares are tendered by shareholders. A special tender offer may be consummated only if (i) at least 5% of the voting power attached to our outstanding shares will be acquired by the offeror and (ii) the number of shares tendered in the offer exceeds the number of shares whose holders objected to the offer.
If a special tender offer is accepted, then the purchaser or any person or entity controlling it or under common control with the purchaser or such controlling person or entity may not make a subsequent tender offer for the purchase of shares of the target company and may not enter into a merger with the target company for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect such an offer or merger in the initial special tender offer.
Merger
The Israeli Companies Law permits merger transactions if approved by each party’s board of directors and, unless certain requirements described under the Israeli Companies Law are met, a majority of each party’s shares voted on the proposed merger at a shareholders’ meeting called with at least 35 days’ prior notice.
116
For purposes of the shareholder vote, unless a court rules otherwise, the merger will not be deemed approved if a majority of the shares represented at the shareholders meeting that are held by parties other than the other party to the merger, or by any person who holds 25% or more of the outstanding shares or the right to appoint 25% or more of the directors of the other party, vote against the merger. If the transaction would have been approved but for the separate approval of each class or the exclusion of the votes of certain shareholders as provided above, a court may still approve the merger upon the request of holders of at least 25% of the voting rights of a company, if the court holds that the merger is fair and reasonable, taking into account the value of the parties to the merger and the consideration offered to the shareholders.
Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of any of the parties to the merger, and may further give instructions to secure the rights of creditors.
In addition, a merger may not be completed unless at least 50 days have passed from the date that a proposal for approval of the merger was filed by each party with the Israeli Registrar of Companies and 30 days have passed from the date the merger was approved by the shareholders of each party.
Antitakeover Measures
The Israeli Companies Law allows us to create and issue shares having rights different from those attached to our ordinary shares, including shares providing certain preferred rights, distributions or other matters and shares having preemptive rights. As of the date of this Annual Report on Form 20-F, we do not have any authorized or issued shares other than our ordinary shares. In the future, if we do create and issue a class of shares other than ordinary shares, such class of shares, depending on the specific rights that may be attached to them, may delay or prevent a takeover or otherwise prevent our shareholders from realizing a potential premium over the market value of their ordinary shares. The authorization of a new class of shares will require an amendment to our Amended and Restated Articles of Association which requires the prior approval of the holders of a majority of our shares at a general meeting. In addition, the rules and regulations of the TASE also limit the terms permitted with respect to a new class of shares and prohibit any such new class of shares from having voting rights. Shareholders voting in such meeting will be subject to the restrictions provided in the Israeli Companies Law as described above.
Borrowing Powers
Under the Israeli Companies Law and our Amended and Restated Articles of Association, our board of directors may exercise all powers and take all actions that are not required under law or under our amended and restated articles of association to be exercised or taken by our shareholders or other corporate bodies, including the power to borrow money for company purposes.
Changes in Capital
Our Amended and Restated Articles of Association enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the Israeli Companies Law and must be approved by a resolution duly passed by our shareholders at a general meeting by voting on such change in the capital. In addition, transactions that have the effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings or profits and, in certain circumstances, an issuance of shares for less than their nominal value, require the approval of both our board of directors and an Israeli court.
C. Material Contracts.
The following are summary descriptions of certain material agreements to which we are a party. The descriptions provided below do not purport to be complete and are qualified in their entirety by the complete agreements, which may be attached as exhibits to this Annual Report on Form 20-F.
License Agreements
See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”.
117
OphthaliX Merger Agreement
See “Item 7. Major Shareholders and Related Party Transactions—B. Related Party Transactions”.
Employment and Consulting Agreements
See “Item 6. Directors, Senior Management and Employees—B. Compensation—Employment and Consulting Agreements”.
D. Exchange Controls
There are no Israeli government laws, decrees or regulations that restrict or that affect our export or import of capital or the remittance of dividends, interest or other payments to non-resident holders of our securities, including the availability of cash and cash equivalents for use by us and our wholly-owned subsidiaries, except or otherwise as set forth under “Item 10. Additional Information—E. Taxation.”
E. Taxation
Certain Israeli Tax Considerations
The following is a summary of the material Israeli tax laws applicable to us. This section also contains a discussion of material Israeli tax consequences concerning the ownership and disposition of our ordinary shares. This summary does not discuss all the aspects of Israeli tax law that may be relevant to a particular investor in light of his or her personal investment circumstances or to some types of investors subject to special treatment under Israeli law. Examples of this kind of investor include residents of Israel or traders in securities who are subject to special tax regimes not covered in this discussion. Because certain parts of this discussion are based on new tax legislation that has not yet been subject to judicial or administrative interpretation, we cannot assure you that the appropriate tax authorities or the courts will accept the views expressed in this discussion. The discussion does not cover all possible tax consequences.
You are urged to consult your own tax advisor as to the Israeli and other tax consequences of the purchase, ownership and disposition of our ADSs, including, in particular, the effect of any non-Israeli, state or local taxes.
General Corporate Tax Structure in Israel
Israeli companies are generally subject to corporate tax, which has decreased in recent years, from a rate of 26.5% in 2014 and 2015 to 25% in 2016 and to 24% in 2017 to 23% for 2018 and 2019. However, the effective corporate tax rate payable by a company that derives income from an Approved Enterprise, a Privileged Enterprise or a Preferred Enterprise (as discussed below) may be considerably less. Capital gains generated by an Israeli company are generally subject to tax at the corporate tax rate.
In 2006, transfer pricing regulations came into force, following the introduction of Section 85A of the Israeli Tax Ordinance under Amendment 132. The transfer pricing rules require that cross-border transactions between related parties be carried out implementing an arms’ length principle based on a transfer pricing study and reported and taxed accordingly.
Pre-Ruling from the Israeli Income Tax Authorities
In connection with the spin-off, we received a pre-ruling decision from the Israeli Income Tax Authority which confirms: (i) that the grant of the license to Eye-Fite is not liable for tax pursuant to the provisions of section 104a to the Income Tax Ordinance (New Version), 1961, or the Ordinance; (ii) that OphthaliX is considered the receiving company pursuant to section 103c(7)(b) to the Ordinance; (iii) that the sale of Eye-Fite shares to OphthaliX as consideration for OphthaliX shares does not create liability for tax pursuant to the provisions of section 103t to the Ordinance, or change in structure; and (iv) the date for the change in structure was determined. According to the tax pre-ruling, the date of change in structure shall also be the date of exchange of shares with respect to the spin-off and notification to the tax assessor. We and Eye-Fite presented to the tax assessor and the merger and spin-off department of the tax assessor the forms required by the Ordinance and the regulations thereunder. The tax pre-ruling further provides that the grant of a license to Eye-Fite as consideration for the issuance of Eye-Fite shares to us does not create liability for tax pursuant to the provisions of section 104a to the Ordinance.
118
According to the pre-ruling, we must not sell more than 10% of our common stock holdings in OphthaliX issued in connection with the change in structure for at least two years from the date of the change (i.e., November 21, 2011), OphthaliX must not sell more than 10% of its ordinary share holdings in Eye-Fite received in connection with the change in structure for at least two years from the date of the change and Eye-Fite must retain the assets received from us in connection with the change in structure for at least two years from the date of the change.
The shares of Eye-Fite which were transferred to OphthaliX in connection with the change in structure will be held in escrow. The sale of these shares will be deemed as a sale by an Israeli company and will be taxed accordingly. The trustee will withhold tax at the source.
The shares of OphthaliX which were transferred to us in connection with the change in structure will be held in escrow. The sale of these shares will be deemed as a sale by an Israeli company and will be taxed accordingly. The trustee will withhold tax at the source.
Any dividend distributed by Eye-Fite to OphthaliX will be taxed in Israel in accordance with paragraph 125(b)5 of the Israeli Tax Ordinance.
A description of the terms of the pre-ruling is also included in the notes to the financial statements.
Tax Benefits and Grants for Research and Development
Israeli tax law allows, under certain conditions, a tax deduction for research and development expenditures, including capital expenditures, for the year in which they are incurred. These expenses must relate to scientific research and development projects and must be approved by the Office of the Chief Scientist, or the OCS, of the relevant Israeli government ministry, determined by the field of research. Furthermore, the research and development must be for the promotion of the company and carried out by or on behalf of the company seeking such tax deduction. The amount of such deductible expenses is reduced by the sum of any funds received through government grants for the funding of the scientific research and development projects. No deduction under these research and development deduction rules is allowed if such deduction is related to an expense invested in an asset depreciable under the general depreciation rules of the Tax Ordinance. Expenditures not so approved are deductible in equal amounts over three years.
On a yearly basis, we evaluate the applicability of the above tax deduction for research and development expenditures and, based on our evaluation, determine whether to apply to the OCS for approval of a tax deduction. There can be no assurance that any application for a tax deduction will be accepted.
Taxation of our Shareholders
Capital Gains Taxes Applicable to Non-Israeli Resident Shareholders. Shareholders that are not Israeli residents are generally exempt from Israeli capital gains tax on any gains derived from the sale, exchange or disposition of our ordinary shares, provided that such shareholders did not acquire their shares prior to our initial public offering on the TASE and such gains were not derived from a permanent establishment or business activity of such shareholders in Israel. However, non-Israeli corporations will not be entitled to the foregoing exemptions if an Israeli resident (i) has a controlling interest of 25% or more in such non-Israeli corporation or (ii) is the beneficiary of or is entitled to 25% or more of the revenues or profits of such non-Israeli corporation, whether directly or indirectly.
119
In addition, under the U.S.-Israel Income Tax Treaty, 1995, or the U.S.-Israel Tax Treaty, the sale, exchange or disposition of our ordinary shares by a shareholder who is a U.S. resident (for purposes of the U.S.-Israel Tax Treaty) holding the shares as a capital asset is exempt from Israeli capital gains tax unless either (i) the shareholder holds, directly or indirectly, shares representing 10% or more of our voting capital during any part of the 12-month period preceding such sale, exchange or disposition or (ii) the capital gains arising from such sale are attributable to a permanent establishment of the shareholder located in Israel. In either case, the sale, exchange or disposition of the shares would be subject to Israeli tax, to the extent applicable; however, under the U.S.-Israel Tax Treaty, the U.S. resident would be permitted to claim a credit for the tax against the U.S. federal income tax imposed with respect to the sale, exchange or disposition, subject to the limitations in U.S. laws applicable to foreign tax credits. The U.S.-Israel Tax Treaty does not relate to U.S. state or local taxes.
Shareholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding at source at the time of sale.
Taxation of Non-Israeli Shareholders on Receipt of Dividends. Non-residents of Israel are generally subject to Israeli income tax on the receipt of dividends paid on our ordinary shares at the rate of 20%, which tax will be withheld at the source, unless a different rate is provided in a tax treaty between Israel and the shareholder’s country of residence. With respect to a person who is a “substantial shareholder” at the time receiving the dividend or on any date in the 12 months preceding such date, the applicable tax rate is 25%. A “substantial shareholder” is generally a person who alone, or together with his relative or another person who collaborates with him on a permanent basis, holds, directly or indirectly, at least 10% of any of the “means of control” of the corporation. “Means of control” generally include the right to vote, receive profits, nominate a director or an officer, receive assets upon liquidation, or order someone who holds any of the aforesaid rights how to act, and all regardless of the source of such right. Under the U.S.-Israel Tax Treaty, the maximum rate of tax withheld in Israel on dividends paid to a holder of our ordinary shares who is a U.S. resident (for purposes of the U.S.-Israel Tax Treaty) is 25%. However, generally, the maximum rate of withholding tax on dividends that are paid to a U.S. corporation holding 10% or more of our outstanding voting capital throughout the tax year in which the dividend is distributed as well as the previous tax year is 12.5%.
A non-resident of Israel who receives dividends from which tax was withheld is generally exempt from the duty to file returns in Israel in respect of such income, provided such income was not derived from a business conducted in Israel by the taxpayer, and the taxpayer has no other taxable sources of income in Israel.
Taxation of Israeli Shareholders on Receipt of Dividends
Residents of Israel are generally subject to Israeli income tax on the receipt of dividends paid on our ordinary shares at the rate of 25%, which tax will be withheld at the source. With respect to a person who is a “substantial shareholder” at the time of receiving the dividend or on any date within the 12 months preceding such date, the applicable tax rate is 30%.
U.S. Federal Income Tax Consequences
The following is a general summary of certain material U.S. federal income tax consequences relating to the purchase, ownership and disposition of our ordinary shares and ADSs by U.S. Holders (as defined below) that hold such ordinary shares or ADSs as capital assets (generally, property held for investment). This summary is based on the Internal Revenue Code of 1986, as amended, or the Code, the regulations of the U.S. Department of the Treasury issued pursuant to the Code, or the Treasury Regulations, administrative and judicial interpretations thereof, and the U.S.-Israel Income Tax Treaty, all as in effect on the date hereof and all of which are subject to change, possibly with retroactive effect, or to different interpretation. No ruling has been sought from the Internal Revenue Service, or IRS, with respect to any United States federal income tax consequences described below, and there can be no assurance that the IRS or a court will not take a contrary position. This summary does not address all of the tax considerations that may be relevant to specific U.S. Holders in light of their particular circumstances or to U.S. Holders subject to special treatment under U.S. federal income tax law, such as (1) a bank, life insurance company, regulated investment company, or other financial institution or “financial services entity”; (2) a broker or dealer in securities or foreign currency; (3) a person who acquired our ordinary shares or ADSs in connection with employment or other performance of services; (4) a U.S. Holder that is subject to the U.S. alternative minimum tax; (5) a U.S. Holder that holds our ordinary shares or ADSs as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction or other risk-reduction transaction for U.S. federal income tax purposes; (6) a tax-exempt entity; (7) real estate investment trusts; (8) a U.S. Holder that expatriates out of the United States or a former long-term resident of the United States; or (9) a U.S. Holder having a functional currency other than the U.S. dollar. This discussion does not address the U.S. federal income tax treatment of a U.S. Holder that owns, directly or constructively, at any time, ordinary shares or ADSs representing 10% or more of our voting power or value. Additionally, this summary does not address any U.S. state or local or non-U.S. tax considerations or any U.S. federal estate, gift or alternative minimum tax considerations or any U.S. federal tax consequences other than U.S. federal income tax consequences.
120
As used in this summary, the term “U.S. Holder” means a beneficial owner of our ordinary shares or ADSs that is, for U.S. federal income tax purposes, (i) an individual citizen or resident of the United States, (ii) a corporation, or other entity taxable as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof, or the District of Columbia, (iii) an estate the income of which is subject to U.S. federal income tax regardless of its source or (iv) a trust with respect to which a court within the United States is able to exercise primary supervision over its administration and one or more U.S. persons have the authority to control all of its substantial decisions, or that has a valid election in effect under applicable Treasury Regulations to be treated as a “United States person.”
If an entity or arrangement treated as a partnership for U.S. federal income tax purposes holds our ordinary shares or ADSs, the tax treatment of such partnership and each partner thereof will generally depend upon the status and activities of the partnership and such partner. A holder that is treated as a partnership for U.S. federal income tax purposes should consult its own tax advisor regarding the U.S. federal income tax considerations applicable to it and its partners of the purchase, ownership and disposition of its ordinary shares or ADSs.
This summary is not intended to be, and should not be considered to be, legal or tax advice. Prospective investors should be aware that this summary does not address the tax consequences to investors who are not U.S. Holders, except to the limited extent discussed below. Prospective investors should consult their own tax advisors as to the particular tax considerations applicable to them relating to the purchase, ownership and disposition of their ordinary shares or ADSs, including the applicability of U.S. federal, state and local tax laws and non-U.S. tax laws.
Taxation of U.S. Holders
The discussions under “— Distributions” and under “— Sale, Exchange or Other Disposition of Ordinary Shares and ADSs” below assumes that we will not be treated as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. Based on our analysis of our income, assets, and operations, we do not believe that we were a PFIC for 2018. Because the PFIC determination is highly fact intensive, there can be no assurance that we will not be a PFIC for 2019 or for any other taxable year. For a discussion of the rules that would apply if we are treated as a PFIC, see the discussion under “— Passive Foreign Investment Company.”
Distributions. We have no current plans to pay dividends. To the extent we pay any dividends, a U.S. Holder will be required to include in gross income as a taxable dividend the amount of any distributions made on the ordinary shares or ADSs, including the amount of any Israeli taxes withheld, to the extent that those distributions are paid out of our current or accumulated earnings and profits as determined for U.S. federal income tax purposes. Any distributions in excess of our earnings and profits will be applied against and will reduce the U.S. Holder’s tax basis in its shares or ADSs and to the extent they exceed that tax basis, will be treated as gain from the sale or exchange of those shares or ADSs. If we were to pay dividends, we expect to pay such dividends in NIS with respect to the shares and in U.S. dollars with respect to ADSs. A dividend paid in NIS, including the amount of any Israeli taxes withheld, will be includible in a U.S. Holder’s income as a U.S. dollar amount calculated by reference to the exchange rate in effect on the date such dividend is received, regardless of whether the payment is in fact converted into U.S. dollars. If the dividend is converted to U.S. dollars on the date of receipt, a U.S. Holder generally will not recognize a foreign currency gain or loss. However, if the U.S. Holder converts the NIS into U.S. dollars on a later date, the U.S. Holder must include, in computing its income, any gain or loss resulting from any exchange rate fluctuations. The gain or loss will be equal to the difference between (i) the U.S. dollar value of the amount included in income when the dividend was received and (ii) the amount received on the conversion of the NIS into U.S. dollars. Such gain or loss will generally be ordinary income or loss and United States source for U.S. foreign tax credit purposes. U.S. Holders should consult their own tax advisors regarding the tax consequences to them if we pay dividends in NIS or any other non-U.S. currency.
121
Subject to certain significant conditions and limitations, including potential limitations under the U.S.-Israel Tax Treaty, any Israeli taxes paid on or withheld from distributions from us and not refundable to a U.S. Holder may be credited against the investor’s U.S. federal income tax liability or, alternatively, may be deducted from the investor’s taxable income. The election to credit or deduct foreign taxes is made on a year-by-year basis and applies to all foreign taxes paid by a U.S. Holder or withheld from a U.S. Holder that year. Dividends paid on the ordinary shares generally will constitute income from sources outside the United States and be categorized as “passive category income” or, in the case of some U.S. Holders, as “general category income” for U.S. foreign tax credit purposes.
Because the rules governing foreign tax credits are complex, U.S. Holders should consult their own tax advisor regarding the availability of foreign tax credits in their particular circumstances.
Dividends paid on the ordinary shares and ADSs will not be eligible for the “dividends-received” deduction generally allowed to corporate U.S. Holders with respect to dividends received from U.S. corporations.
Certain distributions treated as dividends that are received by an individual U.S. Holder from “qualified foreign corporations” generally qualify for a 20% tax rate so long as certain holding period and other requirements are met. A non-U.S. corporation (other than a corporation that is treated as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation (i) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information program, or (ii) with respect to any dividend it pays on stock (or ADSs in respect of such stock) which is readily tradable on an established securities market in the United States. Dividends paid by us in a taxable year in which we are not a PFIC and with respect to which we were not a PFIC in the preceding taxable year are expected to be eligible for the 20% tax rate, although we can offer no assurances in this regard. However, any dividend paid by us in a taxable year in which we are a PFIC or were a PFIC in the preceding taxable year will be subject to tax at regular ordinary income rates. Because the PFIC determination is highly fact intensive, there can be no assurance that we will not be a PFIC in 2018 or in any other taxable year. The additional 3.8% “net investment income tax” (described below) may apply to dividends received by certain U.S. Holders who meet certain modified adjusted gross income thresholds.
Sale, Exchange or Other Disposition of Ordinary Shares and ADSs. Subject to the discussion under “— Passive Foreign Investment Company” below, a U.S. Holder generally will recognize capital gain or loss upon the sale, exchange or other taxable disposition of our ordinary shares or ADSs in an amount equal to the difference between the amount realized on the sale, exchange or other disposition and the U.S. Holder’s adjusted tax basis in such shares. This capital gain or loss will be long-term capital gain or loss if the U.S. Holder’s holding period in our ordinary shares exceeds one year. Preferential tax rates for long-term capital gain (currently, with a maximum rate of 20%) will apply to individual U.S. Holders. The deductibility of capital losses is subject to limitations. The gain or loss will generally be income or loss from sources within the United States for U.S. foreign tax credit purposes, subject to certain exceptions in U.S.-Israel Tax Treaty. The additional 3.8% “net investment income tax” (described below) may apply to gains recognized upon the sale, exchange, or other taxable disposition of our ordinary shares or ADSs by certain U.S. Holders who meet certain modified adjusted gross income thresholds.
U.S. Holders should consult their own tax advisors regarding the U.S. federal income tax consequences of receiving currency other than U.S. dollars upon the disposition of their ordinary shares or ADSs.
Passive Foreign Investment Company
In general, a corporation organized outside the United States will be treated as a PFIC for U.S. federal income tax purposes in any taxable year in which either (i) at least 75% of its gross income is “passive income” or (ii) on average at least 50% of its assets (by value) produce passive income or are held for the production of passive income. Passive income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in the public offering. Assets that produce or are held for the production of passive income include cash, even if held as working capital or raised in a public offering, marketable securities and other assets that may produce passive income. In determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
122
Under the tests described above, whether or not we are a PFIC will be determined annually based upon the composition of our income and the composition and valuation of our assets, all of which are subject to change.
Based on our analysis of our income, assets, and operations, we do not believe that we were a PFIC for 2018. Because the PFIC determination is highly fact intensive, there can be no assurance that we will not be a PFIC in 2019 or in any other taxable year.
Default PFIC Rules. If we are a PFIC for any tax year, a U.S. Holder who does not make a timely QEF election or a mark-to-market election, referred to in this disclosure as a “Non-Electing U.S. Holder,” will be subject to special rules with respect to (i) any “excess distribution” (generally, the portion of any distributions received by the Non-Electing U.S. Holder on the ordinary shares or ADSs in a taxable year in excess of 125% of the average annual distributions received by the Non-Electing U.S. Holder in the three preceding taxable years, or, if shorter, the Non-Electing U.S. Holder’s holding period for the ordinary shares or ADSs), and (ii) any gain realized on the sale or other disposition of such ordinary shares or ADSs. Under these rules:
| ● | the excess distribution or gain would be allocated ratably over the Non-Electing U.S. Holder’s holding period for such ordinary shares or ADSs; |
| ● | the amount allocated to the current taxable year and any year prior to us becoming a PFIC would be taxed as ordinary income; and |
| ● | the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. |
If a Non-Electing U.S. Holder who is an individual dies while owning our ordinary shares or ADSs, the Non-Electing U.S. Holder’s successor would be ineligible to receive a step-up in tax basis of such ordinary shares or ADSs. Non-Electing U.S. Holders should consult their tax advisors regarding the application of the “net investment income tax” (described below) to their specific situation.
To the extent a distribution on our ordinary shares or ADSs does not constitute an excess distribution to a Non-Electing U.S. Holder, such Non-Electing U.S. Holder generally will be required to include the amount of such distribution in gross income as a dividend to the extent of our current or accumulated earnings and profits (as determined for U.S. federal income tax purposes) that are not allocated to excess distributions. The tax consequences of such distributions are discussed above under “— Taxation of U.S. Holders — Distributions.” Each U.S. Holder is encouraged to consult its own tax advisor with respect to the appropriate U.S. federal income tax treatment of any distribution on our ordinary shares.
If we are treated as a PFIC for any taxable year during the holding period of a Non-Electing U.S. Holder, we will continue to be treated as a PFIC for all succeeding years during which the Non-Electing U.S. Holder is treated as a direct or indirect Non-Electing U.S. Holder even if we are not a PFIC for such years. A U.S. Holder is encouraged to consult its tax advisor with respect to any available elections that may be applicable in such a situation, including the “deemed sale” election of Code Section 1298(b)(1) (which will be taxed under the adverse tax rules described above).
123
We may invest in the equity of foreign corporations that are PFICs or may own subsidiaries that own PFICs. If we are classified as a PFIC, under attribution rules U.S. Holders will be subject to the PFIC rules with respect to their indirect ownership interests in such PFICs, such that a disposition of the shares of the PFIC or receipt by us of a distribution from the PFIC generally will be treated as a deemed disposition of such shares or the deemed receipt of such distribution by the U.S. Holder, subject to taxation under the PFIC rules. There can be no assurance that a U.S. Holder will be able to make a QEF election or a mark-to-market election with respect to PFICs in which we invest. Each U.S. Holder is encouraged to consult its own tax advisor with respect to tax consequences of an investment by us in a corporation that is a PFIC.
QEF Election. One way in which certain of the adverse consequences of PFIC status can be mitigated is for a U.S. Holder to make a QEF election. A U.S. Holder who makes a timely QEF election, referred to in this disclosure as an “Electing U.S. Holder,” with respect to us must report for U.S. federal income tax purposes his pro rata share of our ordinary earnings and net capital gain, if any, for our taxable year that ends with or within the taxable year of the Electing U.S. Holder. The “net capital gain” of a PFIC is the excess, if any, of the PFIC’s net long-term capital gains over its net short-term capital losses. The amount so included in income generally will be treated as ordinary income to the extent of such Electing U.S. Holder’s allocable share of the PFIC’s ordinary earnings and as long-term capital gain to the extent of such Electing U.S. Holder’s allocable share of the PFIC’s net capital gains. Such Electing U.S. Holder generally will be required to translate such income into U.S. dollars based on the average exchange rate for the PFIC’s taxable year with respect to the PFIC’s functional currency. Such income generally will be treated as income from sources outside the United States for U.S. foreign tax credit purposes. Amounts previously included in income by such Electing U.S. Holder under the QEF rules generally will not be subject to tax when they are distributed to such Electing U.S. Holder. The Electing U.S. Holder’s tax basis in our ordinary shares or ADSs generally will increase by any amounts so included under the QEF rules and decrease by any amounts not included in income when distributed.
An Electing U.S. Holder will be subject to U.S. federal income tax on such amounts for each taxable year in which we are a PFIC, regardless of whether such amounts are actually distributed to such Electing U.S. Holder. However, an Electing U.S. Holder may, subject to certain limitations, elect to defer payment of current U.S. federal income tax on such amounts, subject to an interest charge. If an Electing U.S. Holder is an individual, any such interest will be treated as non-deductible “personal interest.”
Any net operating losses or net capital losses of a PFIC will not pass through to the Electing U.S. Holder and will not offset any ordinary earnings or net capital gain of a PFIC recognized by Electing U.S. Holder in subsequent years.
So long as an Electing U.S. Holder’s QEF election with respect to us is in effect with respect to the entire holding period for our ordinary shares or ADSs, any gain or loss recognized by such Electing U.S. Holder on the sale, exchange or other disposition of such shares or ADSs generally will be long-term capital gain or loss if such Electing U.S. Holder has held such shares or ADSs for more than one year at the time of such sale, exchange or other disposition. Preferential tax rates for long-term capital gain (currently, a maximum rate of 20%) will apply to individual U.S. Holders. The deductibility of capital losses is subject to limitations.
In general, a U.S. Holder must make a QEF election on or before the due date for filing its income tax return for the first year to which the QEF election is to apply. A U.S. Holder makes a QEF election by completing the relevant portions of and filing IRS Form 8621 in accordance with the instructions thereto. Upon request, we will annually furnish U.S. Holders with information needed in order to complete IRS Form 8621 (which form would be required to be filed with the IRS on an annual basis by the U.S. Holder) and to make and maintain a valid QEF election for any year in which we or any of our subsidiaries that we control is a PFIC. There is no assurance, however, that we will have timely knowledge of our status as a PFIC, or that the information that we provide will be adequate to allow U.S. Holders to make a QEF election. A QEF election will not apply to any taxable year during which we are not a PFIC, but will remain in effect with respect to any subsequent taxable year in which we become a PFIC. Each U.S. Holder should consult its own tax advisor with respect to the advisability of, the tax consequences of, and the procedures for making a QEF election with respect to us.
124
Mark-to-Market Election. Alternatively, if our ordinary shares or ADSs are treated as “marketable stock,” a U.S. Holder would be allowed to make a “mark-to-market” election with respect to our ordinary shares or ADSs, provided the U.S. Holder completes and files IRS Form 8621 in accordance with the relevant instructions and related Treasury Regulations. If that election is made, the U.S. Holder generally would include as ordinary income in each taxable year the excess, if any, of the fair market value of our ordinary shares or ADSs at the end of the taxable year over such holder’s adjusted tax basis in such shares or ADSs. The U.S. Holder would also be permitted an ordinary loss in respect of the excess, if any, of the U.S. Holder’s adjusted tax basis in our ordinary shares or ADSs over their fair market value at the end of the taxable year, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. A U.S. Holder’s tax basis in our ordinary shares or ADSs would be adjusted to reflect any such income or loss amount. Gain realized on the sale, exchange or other disposition of our ordinary shares or ADSs would be treated as ordinary income, and any loss realized on the sale, exchange or other disposition of our ordinary shares or ADSs would be treated as ordinary loss to the extent that such loss does not exceed the net mark-to-market gains previously included in income by the U.S. Holder, and any loss in excess of such amount will be treated as capital loss. Amounts treated as ordinary income will not be eligible for the favorable tax rates applicable to qualified dividend income or long-term capital gains.
Generally, stock will be considered marketable stock if it is “regularly traded” on a “qualified exchange” within the meaning of applicable Treasury Regulations. A class of stock is regularly traded on an exchange during any calendar year during which such class of stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. To be marketable stock, our ordinary shares and ADSs must be regularly traded on a qualifying exchange (i) in the United States that is registered with the SEC or a national market system established pursuant to the Exchange Act or (ii) outside the United States that is properly regulated and meets certain trading, listing, financial disclosure and other requirements. Our ordinary shares should constitute “marketable stock” as long as they remain listed on the OTC and/or the NYSE American and are regularly traded. Our ADSs will be listed on the OTC and/or the NYSE American. While we believe that our ADSs may be treated as marketable stock for purposes of the PFIC rules so long as they are listed on the OTC and/or the NYSE American and are regularly traded, the IRS has not provided a list of the exchanges that meet the foregoing requirements and thus no assurance can be provided that our ADSs will be (or will remain) treated as marketable stock for purposes of the PFIC rules.
A mark-to-market election will not apply to our ordinary shares or ADSs held by a U.S. Holder for any taxable year during which we are not a PFIC, but will remain in effect with respect to any subsequent taxable year in which we become a PFIC. Such election will not apply to any PFIC subsidiary that we own. Each U.S. Holder is encouraged to consult its own tax advisor with respect to the availability and tax consequences of a mark-to-market election with respect to our ordinary shares and ADSs.
In addition, U.S. Holders should consult their tax advisors regarding the IRS information reporting and filing obligations that may arise as a result of the ownership of ordinary shares in a PFIC, including IRS Form 8621, Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund.
The U.S. federal income tax rules relating to PFICs, QEF elections, and mark-to market elections are complex. U.S. Holders are urged to consult their own tax advisors with respect to the purchase, ownership and disposition of our ordinary shares or ADSs, any elections available with respect to such shares or ADSs and the IRS information reporting obligations with respect to the purchase, ownership and disposition of our ordinary shares or ADSs.
Tax Consequences for Non-U.S. Holders of Ordinary Shares or ADSs
Except as provided below, an individual, corporation, estate or trust that is not a U.S. Holder, referred to below as a non-U.S. Holder, generally will not be subject to U.S. federal income or withholding tax on the payment of dividends on, and the proceeds from the disposition of, our ordinary shares or ADSs.
A non-U.S. Holder may be subject to U.S. federal income tax on a dividend paid on our ordinary shares or ADSs or gain from the disposition of our ordinary shares or ADSs if: (1) such item is effectively connected with the conduct by the non-U.S. Holder of a trade or business in the United States and, if required by an applicable income tax treaty is attributable to a permanent establishment or fixed place of business in the United States; or (2) in the case of a disposition of our ordinary shares or ADSs, the individual non-U.S. Holder is present in the United States for 183 days or more in the taxable year of the disposition and other specified conditions are met.
In general, non-U.S. Holders will not be subject to backup withholding with respect to the payment of dividends on our ordinary shares or ADSs if payment is made through a paying agent, or office of a foreign broker outside the United States. However, if payment is made in the United States or by a U.S. related person, non-U.S. Holders may be subject to backup withholding, unless the non-U.S. Holder provides an applicable IRS Form W-8 (or a substantially similar form) certifying its foreign status, or otherwise establishes an exemption.
The amount of any backup withholding from a payment to a non-U.S. Holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
125
Certain Reporting Requirements
Certain U.S. Holders are required to file IRS Form 926, Return by U.S. Transferor of Property to a Foreign Corporation, and certain U.S. Holders may be required to file IRS Form 5471, Information Return of U.S. Persons With Respect to Certain Foreign Corporations, reporting transfers of cash or other property to us and information relating to the U.S. Holder and us. Substantial penalties may be imposed upon a U.S. Holder that fails to comply. See also the discussion regarding Form 8621, Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund, above.
In addition, certain U.S. Holders must report information on IRS Form 8938, Statement of Specified Foreign Financial Assets, with respect to their investments in certain “foreign financial assets,” which would include an investment in our ordinary shares, if the aggregate value of all of those assets exceeds $50,000 on the last day of the taxable year (and in some circumstances, a higher threshold). This reporting requirement applies to individuals and certain U.S. entities.
U.S. Holders who fail to report required information could become subject to substantial penalties. U.S. Holders should consult their tax advisors regarding the possible implications of these reporting requirements arising from their investment in our ordinary shares.
Backup Withholding Tax and Information Reporting Requirements
Generally, information reporting requirements will apply to distributions on our ordinary shares or ADSs or proceeds on the disposition of our ordinary shares or ADSs paid within the United States (and, in certain cases, outside the United States) to U.S. Holders other than certain exempt recipients, such as corporations. Furthermore, backup withholding (currently at 24%) may apply to such amounts if the U.S. Holder fails to (i) provide a correct taxpayer identification number, (ii) report interest and dividends required to be shown on its U.S. federal income tax return, or (iii) make other appropriate certifications in the required manner. U.S. Holders who are required to establish their exempt status generally must provide such certification on IRS Form W-9.
Backup withholding is not an additional tax. Amounts withheld as backup withholding from a payment may be credited against a U.S. Holder’s U.S. federal income tax liability and such U.S. Holder may obtain a refund of any excess amounts withheld by filing the appropriate claim for refund with the IRS and furnishing any required information in a timely manner.
Tax on Net Investment Income
Certain U.S. persons, including individuals, estates and trusts are generally subject to an additional 3.8% Medicare tax. For individuals, the additional Medicare tax applies to the lesser of (i) “net investment income” or (ii) the excess of “modified adjusted gross income” over $200,000 ($250,000 if married and filing jointly or $125,000 if married and filing separately). “Net investment income” generally equals the taxpayer’s gross investment income reduced by the deductions that are allocable to such income. Investment income generally includes passive income such as interest, dividends, annuities, royalties, rents, and capital gains. U.S. Holders are urged to consult their own tax advisors regarding the implications of the additional Medicare tax resulting from their ownership and disposition of our shares or ADSs.
U.S. Holders should consult their own tax advisors concerning the tax consequences relating to the purchase, ownership and disposition of our ordinary shares or ADSs.
126
F. Dividends and Paying Agents.
Not applicable.
G. Statements by Experts.
Not applicable.
H. Documents on Display.
We are subject to the information reporting requirements of the Exchange Act, applicable to foreign private issuers and under those requirements will file reports with the SEC. The SEC maintains an Internet website that contains reports and other information regarding issuers that file electronically with the SEC. You may read and copy this annual report, including the related exhibits and schedules, and any document we file with the SEC at http://www.sec.gov.
As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. However, we will file with the SEC, within 120 days after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm, and will submit to the SEC, on a Form 6-K, unaudited quarterly financial information.
In addition, because our ordinary shares are traded on the TASE, we have filed Hebrew language periodic and immediate reports with, and furnish information to, the TASE and the ISA, as required under Chapter Six of the Israel Securities Law. Copies of our filings with the ISA can be retrieved electronically through the MAGNA distribution site of the ISA (www.magna.isa.gov.il) and the TASE website (www.maya.tase.co.il).
We maintain a corporate website at www.canfite.com. Information contained on, or that can be accessed through, our website does not constitute a part of this Annual Report on Form 20-F. We have included our website address in this Annual Report on Form 20-F solely as an inactive textual reference.
I. Subsidiary Information.
Not applicable.
ITEM 11. Quantitative and Qualitative Disclosures About Market Risk
Market risk is the risk of loss related to changes in market prices, including interest rates and foreign exchange rates, of financial instruments that may adversely impact our consolidated financial position, results of operations or cash flows.
Interest Rate Risk
We do not anticipate undertaking any significant long-term borrowings. At present, our investments consist primarily of cash and cash equivalents. We may invest in investment-grade marketable securities with maturities of up to three years, including commercial paper, money market funds, and government/non-government debt securities. The primary objective of our investment activities is to preserve principal while maximizing the income that we receive from our investments without significantly increasing risk and loss. Our investments are exposed to market risk due to fluctuation in interest rates, which may affect our interest income and the fair market value of our investments, if any. We manage this exposure by performing ongoing evaluations of our investments. Due to the short-term maturities, if any, of our investments to date, their carrying value has always approximated their fair value. If we decide to invest in investments other than cash and cash equivalents, it will be our policy to hold such investments to maturity in order to limit our exposure to interest rate fluctuations.
127
Foreign Currency Exchange Risk
Our foreign currency exposures give rise to market risk associated with exchange rate movements of the U.S dollar, our functional and reporting currency, mainly against the NIS and the euro. Although the U.S dollar is our functional currency, a portion of our expenses are denominated in both NIS and euro and currently all of our revenues are denominated in dollars. Our U.S. dollar and euro expenses consist principally of payments made to sub-contractors and consultants for preclinical studies, clinical trials and other research and development activities, Our NIS expenses consist principally of salary related payments. We anticipate that a sizable portion of our expenses will continue to be denominated in currencies other than the NIS. If the U.S dollar fluctuates significantly against either the NIS or the euro, it may have a negative impact on our results of operations. To date, fluctuations in the exchange rates have not materially affected our results of operations or financial condition for the periods under review.
To date, we have not engaged in hedging transactions. In the future, we may enter into currency hedging transactions to decrease the risk of financial exposure from fluctuations in the exchange rates of our principal operating currencies. These measures, however, may not adequately protect us from the material adverse effects of such fluctuations.
ITEM 12. Description of Securities Other Than Equity Securities
A. Debt Securities.
Not applicable.
B. Warrants and Rights.
Not applicable.
C. Other Securities.
Not applicable.
D. American Depositary Shares
The Bank of New York Mellon, as Depositary, will register and deliver American Depositary Shares, or ADSs. Each ADS will represent two (2) ordinary shares (or a right to receive two (2) ordinary shares) deposited with the principal Tel Aviv office of Bank Hapoalim, as custodian for the Depositary. Each ADS will also represent any other securities, cash or other property which may be held by the Depositary. The Depositary’s corporate trust office at which the ADSs will be administered is located at 101 Barclay Street, New York, New York 10286. The Bank of New York Mellon’s principal executive office is located at One Wall Street, New York, New York 10286.
The form of the deposit agreement for our ADSs and the form of American Depositary Receipt that represents an ADS have been incorporated by reference as exhibits to this Annual Report on Form 20-F.
128
Fees and Expenses
| Persons depositing or withdrawing shares or ADS holders must pay: | For: | ||
| $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) | ● | Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property | |
| ● | Cancellation of ADSs for the purpose of withdrawal, including if the Deposit Agreement terminates | ||
| $.05 (or less) per ADS | ● | Any cash distribution to ADS holders | |
| A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs | ● | Distribution of securities distributed to holders of deposited securities which are distributed by the Depositary to ADS holders | |
| $.05 (or less) per ADSs per calendar year | ● | Depositary services | |
| Registration or transfer fees | ● | Transfer and registration of shares on our share register to or from the name of the Depositary or its agent when you deposit or withdraw shares | |
| Expenses of the Depositary | ● | Cable, telex and facsimile transmissions (when expressly provided in the Deposit Agreement) | |
| ● | Converting foreign currency to U.S. dollars | ||
| Taxes and other governmental charges the Depositary or the custodian have to pay on any ADS or share underlying an ADS, for example, stock transfer taxes, stamp duty or withholding taxes | ● | As necessary | |
| Any charges incurred by the Depositary or its agents for servicing the deposited securities | ● | As necessary | |
The Depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The Depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The Depositary may collect its annual fee for depositary services by deduction from cash distributions, by directly billing investors or by charging the book-entry system accounts of participants acting for them. The Depositary may generally refuse to provide fee-attracting services until its fees for those services are paid.
From time to time, the Depositary may make payments to us to reimburse us for expenses and/or share revenue with us from the fees collected from ADS holders, or waive fees and expenses for services provided, generally relating to costs and expenses arising out of the establishment and maintenance of the ADS program. In performing its duties under the Deposit Agreement, the Depositary may use brokers, dealers or other service providers that are affiliates of the Depositary and that may earn or share fees or commissions.
129
ITEM 13. Defaults, Dividend Arrearages and Delinquencies
Not applicable.
ITEM 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
Not applicable.
ITEM 15. Controls and Procedures
Disclosure controls and procedures
Our management, including our chief executive officer, or CEO, and our chief financial officer, or CFO, are responsible for establishing and maintaining our disclosure controls and procedures (within the meaning of Rule 13a-15(e) of the Exchange Act). These controls and procedures were designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC and that such information is accumulated and communicated to our management, including our CEO and CFO, as appropriate, to allow timely decisions regarding required disclosure. We evaluated these disclosure controls and procedures under the supervision of our CEO and CFO as of December 31, 2018. Based upon that evaluation, our management, including our CEO and CFO, concluded that our disclosure controls and procedures as of December 31, 2018 were effective.
Management’s annual report on internal control over financial reporting
Our management, including our CEO, and our CFO, is responsible for establishing and maintaining adequate internal control over our financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act of 1934, as amended. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Internal control over financial reporting includes policies and procedures that:
| ● | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and asset dispositions; | |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit the preparation of our financial statements in accordance with generally accepted accounting principles; | |
| ● | provide reasonable assurance that receipts and expenditures are made only in accordance with authorizations of our management and board of directors (as appropriate); and | |
| ● | provide reasonable assurance regarding the prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on our financial statements. |
Due to its inherent limitations, internal controls over financial reporting may not prevent or detect misstatements. In addition, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our CEO, and our CFO, we assessed the effectiveness of our internal control over financial reporting as of December 31, 2018 based on the framework for Internal Control-Integrated Framework set forth by The Committee of Sponsoring Organizations of the Treadway Commission (COSO) (2013).
Based on our assessment and this framework, our management concluded that our internal control over financial reporting were effective as of December 31, 2018.
Attestation Report of Registered Public Accounting Firm
Not applicable.
Changes in internal control over financial reporting
There were no changes in our internal control over financial reporting, other than as described above, that occurred during the year ended December 31, 2018 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
130
ITEM 16A. Audit Committee Financial Expert
Our Board of Directors has determined that Guy Regev and Yaacov Goldman are audit committee financial experts, as defined by applicable SEC regulations. Messrs. Regev and Goldman qualified as an “independent director,” as that term is defined under NYSE American rules.
We have adopted a code of ethics, referred to as a Code of Business Conduct, applicable to our directors, officers and all other employees. Our code of ethics is publicly available on our website at www.canfite.com. If we make any amendment to the code of ethics or grant any waivers, including any implicit waiver, from a provision of the code of ethics, which applies to our chief executive officer, chief financial officer, chief accounting officer or controller, or persons performing similar functions, we will disclose the nature of such amendment or waiver on our website.
ITEM 16C. Principal Accountant Fees and Services
The following table sets forth, for each of the years indicated, the fees billed by our independent registered public accounting firm.
| Year Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| Services Rendered | (USD in thousands) | |||||||
| Audit (1) | 115 | 95 | ||||||
| Audit related services (2) | 9 | 15 | ||||||
| Tax | 14 | 10 | ||||||
| All other fees | - | - | ||||||
| Total | 138 | 120 | ||||||
| (1) | Audit fees consist of services that would normally be provided in connection with statutory and regulatory filings or engagements, including services that generally only the independent accountant can reasonably provide. |
| (2) | Audit related services consist of services that were reasonably related to the performance of the audit or reviews of our financial statements and not included under “Audit Fees” above, including, principally, providing consents for registration statement filings. |
Audit Committee Pre-Approval Policies and Procedures
Our audit committee’s specific responsibilities in carrying out its oversight of the quality and integrity of the accounting, auditing and reporting practices of us include the approval of audit and non-audit services to be provided by the external auditor. The audit committee approves in advance the particular services or categories of services to be provided to us during the following yearly period and also sets forth a specific budget for such audit and non-audit services. Additional non-audit services may be pre-approved by the audit committee.
ITEM 16D. Exemptions from the Listing Standards for Audit Committees
Not applicable.
ITEM 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
Not applicable.
131
ITEM 16F. Change in Registrant’s Certifying Accountant
Not applicable.
ITEM 16G. Corporate Governance
We are a foreign private issuer whose ordinary shares are listed on the NYSE American. As such, we are required to comply with U.S. federal securities laws, including the Sarbanes-Oxley Act, and the NYSE American rules, including the NYSE American corporate governance requirements. The NYSE American rules provide that foreign private issuers may follow home country practice in lieu of certain qualitative listing requirements subject to certain exceptions and except to the extent that such exemptions would be contrary to U.S. federal securities laws, so long as the foreign issuer discloses that it does not follow such listing requirement and describes the home country practice followed in its reports filed with the SEC. Below is a concise summary of the significant ways in which our corporate governance practices differ from the corporate governance requirements of NYSE American applicable to domestic U.S. listed companies:
| ● | The NYSE American rules recommend that an issuer have a quorum requirement for shareholders meetings of at least one-third of the outstanding shares of the issuer’s common voting stock. We have chosen to follow home country practice with respect to the quorum requirements of our shareholders meeting and our adjourned shareholders meeting. Our Amended and Restated Articles of Association, as permitted under the Israeli Companies Law and Israeli practice, provide that the quorum requirements for a shareholders meeting are the presence of at least two shareholders who represent at least 25%of the outstanding shares of the issuer’s common voting stock, and in the event of an adjourned meeting, the presence of a minimum of two shareholders present in person. |
| ● | We have chosen to follow our home country practice in lieu of the requirements of the NYSE American rules relating to shareholder approval required prior to the issuance of securities (i) when a stock option or purchase plan is to be established or materially amended or other equity compensation arrangement made or materially amended, pursuant to which stock may be acquired by officers, directors, employees or consultants and (ii) in connection with a transaction, other than a public offering, involving the issuance or potential issuance by the Company of ordinary shares (or their equivalent) equal to 20% or more of the ordinary shares or 20% voting power outstanding before the issuance for or at a price less than the greater of book or market value of the shares. We follow the provisions of the Israeli Companies Law with regard to transactions with our affiliates, i.e., our controlling shareholder and our directors and officers, including private placement transactions. |
ITEM 16H. Mine Safety Disclosure
Not applicable.
132
We have responded to Item 18 in lieu of responding to this item.
Please refer to the financial statements beginning on page F-1.
Index to Exhibits
133
134
| Exhibit No. | Description | |
| 13.1 | Certification by Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002* | |
| 13.2 | Certification by Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002* | |
| 15.1 | Consent of Independent Registered Public Accounting Firm.* | |
| 101 | The following financial information from Can-Fite BioPharma Ltd.’s Annual Report on Form 20-F for the year ended December 31, 2018, formatted in Extensible Business Reporting Language (XBRL): (i) Consolidated Statements of Financial Position, (ii) Consolidated Statements of Comprehensive Loss, (iii) Consolidated Statements of Changes in Equity (Deficiency) (iv) the Consolidated Statements of Cash Flows, and (iv) Notes to Consolidated Financial Statements.* |
| * | Filed Herewith. |
| † | Certain confidential information contained in this exhibit was omitted by means of redacting a portion of the text and replacing it with [...]. This exhibit has been filed separately with the Commission without the redaction pursuant to a Confidential Treatment Request under Rule 24b-2 of the Securities Exchange Act of 1934 and Rule 406 of the Securities Act. |
| (1) | Incorporated herein by reference to the Registration Statement on Form 8-A filed with the SEC on November 15, 2013. |
| (2) | Incorporated herein by reference to Amendment No. 1 to the Draft Registration Statement on Form 20-F filed with the SEC on September 10, 2013. |
| (3) | Incorporated herein by reference to Annual Report on Form 20-F filed with the SEC on March 27, 2015. |
| (4) | Incorporated herein by reference to the Current Report on Form 6-K filed with the SEC on December 4, 2014. |
| (5) | Incorporated herein by reference to the Current Report on Form 6-K filed with the SEC on September 22, 2015. |
| (6) | Incorporated herein by reference to the Current Report on Form 6-K filed with the SEC on October 15, 2015. |
| (7) | Incorporated herein by reference to the Current Report on Form 6-K filed with the SEC on December 7, 2016. |
| (8) | Incorporated herein by reference to the Current Report on Form 6-K filed with the SEC on January 20, 2017. |
| (9) | Incorporated herein by reference to the Annual Report on Form 20- F filed with the SEC on March 30, 2017. |
| (10) | Incorporated herein by reference to the Current Report on Form 8-K filed by OphthaliX, Inc. with the SEC on May 22, 2017. |
| (11) | Incorporated herein by reference to the Current Report on Form 6-K filed with the SEC on March 12, 2018. |
| (12) | Incorporated herein by reference to the Current Report on Form 6-K filed with the SEC on January 22, 2019. |
| (13) | Incorporated herein by reference to Registration Statement on Form F-1 filed with the SEC on February 15, 2019. |
| (14) | Incorporated herein by reference to Annual Report on Form 20-F filed with the SEC on March 28, 2018. |
135
SIGNATURES
The Registrant hereby certifies that it meets all of the requirements for filing on Form 20-F/A and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
| CAN-FITE BIOPHARMA LTD. | ||
| Date: April 2, 2019 | By: | /s/ Pnina Fishman, Ph.D. |
| Pnina Fishman, Ph.D. | ||
| Chief Executive Officer | ||
136
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2018
INDEX
- - - - - - - - - - -
F-1
 |
Kost Forer Gabbay & Kasierer 144 Menachem Begin Road Tel-Aviv 6492102, Israel
|
Tel: +972-3-6232525 Fax: +972-3-5622555 ey.com
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACOUNTING FIRM
To the Shareholders and Board of Directors of
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
Opinion on the Financial Statements
We have audited the accompanying consolidated financial position of Can-Fite Ltd and its subsidiaries (the “Company”) as of December 31, 2018 and 2017, and the related consolidated statements of other comprehensive loss, shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2018, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidation financial statements present fairly, in all material respects, the consolidated financial position of the Company at December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2018, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Kost Forer Gabbay & Kasierer
KOST FORER GABBAY & KASIERER
A Member of Ernst & Young Global
We have served as the Company’s auditor since at least 2001, but we are unable to determine the specific year.
Tel-Aviv, Israel
March 29, 2019
F-2
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
In thousands (except for share and per share data)
| December 31, | ||||||||||
| 2018 | 2017 | |||||||||
| Note | USD | |||||||||
| ASSETS | ||||||||||
| CURRENT ASSETS: | ||||||||||
| Cash and cash equivalents | $ | 3,615 | $ | 3,505 | ||||||
| Other accounts receivables and prepaid expenses | 5 | 4,015 | 3,159 | |||||||
| Short-term investment | 6 | 273 | - | |||||||
| Total current assets | 7,903 | 6,664 | ||||||||
| NON-CURRENT ASSETS: | ||||||||||
| Lease deposit | 2 | 5 | ||||||||
| long-term investment | 6 | - | 917 | |||||||
| Property, plant and equipment, net | 7 | 47 | 28 | |||||||
| Total long-term assets | 49 | 950 | ||||||||
| Total assets | $ | 7,952 | $ | 7,614 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-3
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
In thousands (except for share and per share data)
| December 31, | ||||||||||
| 2018 | 2017 | |||||||||
| Note | USD | |||||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||||
| CURRENT LIABILITIES: | ||||||||||
| Trade payables | $ | 1,071 | $ | 427 | ||||||
| Deferred revenues | 10 | 926 | 330 | |||||||
| Other accounts payable | 8 | 1,122 | 997 | |||||||
| Total current liabilities | 3,119 | 1,754 | ||||||||
| NON-CURRENT LIABILITIES: | ||||||||||
| Deferred revenues | 10 | 1,818 | 846 | |||||||
| Total Long-term liabilities | 1,818 | 846 | ||||||||
| CONTIGENT LIABILITIES AND COMMITMENTS | 10 | |||||||||
| EQUITY ATTRIBUTABLE TO EQUITY HOLDERS OF THE COMPANY: | 11 | |||||||||
| Share capital | 2,635 | 2,123 | ||||||||
| Share premium | 81,668 | 81,104 | ||||||||
| Capital reserve from share-based payment transactions | 5,800 | 5,547 | ||||||||
| Warrants exercisable into shares | 12,408 | 8,815 | ||||||||
| Accumulated other comprehensive income | 1,127 | 1,127 | ||||||||
| Accumulated deficit | (100,623 | ) | (93,702 | ) | ||||||
| Total equity | 3,015 | 5,014 | ||||||||
| Total liabilities and equity | $ | 7,952 | $ | 7,614 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-4
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
In thousands (except for share and per share data)
| Year ended December 31, | ||||||||||||||
| 2018 | 2017 | 2016 | ||||||||||||
| Note | USD | |||||||||||||
| Revenues | 10 | $ | 3,820 | $ | 789 | $ | 165 | |||||||
| Research and development expenses | 13 | 6,075 | 5,106 | 6,115 | ||||||||||
| General and administrative expenses | 14 | 3,159 | 2,868 | 2,733 | ||||||||||
| Operating loss | 5,414 | 7,185 | 8,683 | |||||||||||
| Other income | 1b | - | (769 | ) | - | |||||||||
| Financial expenses | 15 | 1,204 | 621 | 55 | ||||||||||
| Financial income | 15 | (51 | ) | (633 | ) | (374 | ) | |||||||
| Total Financial expense (income), net | 1,153 | (12 | ) | (319 | ) | |||||||||
| Loss before taxes on income | 6,567 | 6,404 | 8,364 | |||||||||||
| Taxes on income | 17 | 4 | 29 | 29 | ||||||||||
| Net loss | 6,571 | 6,433 | 8,393 | |||||||||||
| Other comprehensive loss: | ||||||||||||||
| Amounts that will not be reclassified subsequently to profit or loss: | ||||||||||||||
| Adjustment arising from translating financial statements from functional currency to presentation currency | - | (636 | ) | (119 | ) | |||||||||
| Total other comprehensive loss | - | (636 | ) | (119 | ) | |||||||||
| Total comprehensive loss | $ | 6,571 | $ | 5,797 | $ | 8,274 | ||||||||
| Net loss Attributable to: | ||||||||||||||
| Equity holders of the Company | $ | 6,571 | $ | 6,339 | $ | 8,257 | ||||||||
| Non-controlling interests | - | 94 | 136 | |||||||||||
| 6,571 | 6,433 | 8,393 | ||||||||||||
| Total comprehensive loss attributable to: | ||||||||||||||
| Equity holders of the Company | 6,571 | 5,703 | 8,138 | |||||||||||
| Non-controlling interests | - | 94 | 136 | |||||||||||
| $ | 6,571 | $ | 5,797 | $ | 8,274 | |||||||||
| Net loss per share attributable to equity holders of the Company: | ||||||||||||||
| Basic and diluted net loss per share | 16 | $ | 0.17 | $ | 0.19 | $ | 0.30 | |||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-5
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
In thousands (except for share and per share data)
| Attributable to equity holders of the Company | ||||||||||||||||||||||||||||||||||||||||
| Share capital | Share premium | Capital reserve from share-based payment transactions | Warrants exercisable into shares | Treasury shares | Accumulated other comprehensive income (loss) | Accumulated deficit | Total | Non- controlling interests | Total Equity | |||||||||||||||||||||||||||||||
| USD | ||||||||||||||||||||||||||||||||||||||||
| Balance as of January 1, 2016 | $ | 1,780 | $ | 79,864 | $ | 4,864 | $ | 6,947 | $ | (970 | ) | $ | 232 | $ | (78,966 | ) | $ | 13,751 | $ | 175 | $ | 13,926 | ||||||||||||||||||
| Net loss | - | - | - | - | - | - | (8,257 | ) | (8,257 | ) | (136 | ) | (8,393 | ) | ||||||||||||||||||||||||||
| Loss from defined benefit plans | - | - | - | - | - | 140 | (140 | ) | - | - | - | |||||||||||||||||||||||||||||
| Adjustment arising from translating financial statements from functional currency to presentation currency | - | - | - | - | - | 119 | - | 119 | - | 119 | ||||||||||||||||||||||||||||||
| Total comprehensive loss | - | - | - | - | - | 259 | (8,397 | ) | (8,138 | ) | (136 | ) | (8,274 | ) | ||||||||||||||||||||||||||
| Share-based payments | 3 | - | 303 | - | - | - | - | 306 | 3 | 309 | ||||||||||||||||||||||||||||||
| Balance as of December 31, 2016 | $ | 1,783 | $ | 79,864 | $ | 5,167 | $ | 6,947 | $ | (970 | ) | $ | 491 | $ | (87,363 | ) | $ | 5,919 | $ | 42 | $ | 5,961 | ||||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-6
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
In thousands (except for share and per share data)
| Attributable to equity holders of the Company | ||||||||||||||||||||||||||||||||||||||||
| Share capital | Share premium | Capital reserve from share-based payment transactions | Warrants exercisable into shares | Treasury shares | Accumulated other comprehensive income | Accumulated deficit | Total | Non- controlling interests | Total Equity | |||||||||||||||||||||||||||||||
| USD | ||||||||||||||||||||||||||||||||||||||||
| Balance as of January 1, 2017 | $ | 1,783 | $ | 79,864 | $ | 5,167 | $ | 6,947 | $ | (970 | ) | $ | 491 | $ | (87,363 | ) | $ | 5,919 | $ | 42 | $ | 5,961 | ||||||||||||||||||
| Net loss | - | - | - | - | - | - | (6,339 | ) | (6,339 | ) | (94 | ) | (6,433 | ) | ||||||||||||||||||||||||||
| Adjustment arising from translating financial statements from functional currency to presentation currency | - | - | - | - | - | 636 | - | 636 | - | 636 | ||||||||||||||||||||||||||||||
| Total comprehensive loss | - | - | - | - | - | 636 | (6,339 | ) | (5,703 | ) | (94 | ) | (5,797 | ) | ||||||||||||||||||||||||||
| Issuance of share capital and warrants, net of issue expenses of USD 621 | 330 | 1,993 | 188 | 1,868 | - | - | 4,379 | - | 4,379 | |||||||||||||||||||||||||||||||
| Issuance of share capital | 10 | 85 | - | - | - | - | 95 | - | 95 | |||||||||||||||||||||||||||||||
| Proceeds from sale of subsidiary in previously consolidated subsidiaries | - | (838 | ) | - | - | 970 | - | 132 | 52 | 184 | ||||||||||||||||||||||||||||||
| Share-based payments | - | - | 192 | - | - | - | - | 192 | - | 192 | ||||||||||||||||||||||||||||||
| Balance as of December 31, 2017 | $ | 2,123 | $ | 81,104 | $ | 5,547 | $ | 8,815 | $ | - | $ | 1,127 | $ | (93,702 | ) | $ | 5,014 | $ | - | $ | 5,014 | |||||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-7
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
In thousands (except for share and per share data)
| Attributable to equity holders of the Company | ||||||||||||||||||||||||||||||||||||||||
| Share capital | Share premium | Capital reserve from share-based payment transactions | Warrants exercisable into shares | Treasury shares | Accumulated other comprehensive income (loss) | Accumulated deficit | Total | Non- controlling interests | Total equity | |||||||||||||||||||||||||||||||
| Balance as of January 1, 2018 | $ | 2,123 | $ | 81,104 | $ | 5,547 | $ | 8,815 | $ | - | $ | 1,127 | $ | (93,702 | ) | $ | 5,014 | $ | - | $ | 5,014 | |||||||||||||||||||
| Net loss | - | - | - | - | - | - | (6,571 | ) | (6,571 | ) | - | (6,571 | ) | |||||||||||||||||||||||||||
Cumulative effect of initial adoption of IFRS 15 as of January 1, 2018 (see Note 4) | - | - | - | - | - | - | (350 | ) | (350 | ) | - | (350 | ) | |||||||||||||||||||||||||||
| Issuance of share capital and warrants, net of issuance expenses of USD 613 | 482 | 312 | - | 3,593 | - | - | - | 4,387 | - | 4,387 | ||||||||||||||||||||||||||||||
| Issuance of share capital | 30 | 252 | - | - | - | - | - | 282 | - | 282 | ||||||||||||||||||||||||||||||
| Share-based payment | - | - | 253 | - | - | - | - | 253 | - | 253 | ||||||||||||||||||||||||||||||
| Balance as of December 31, 2018 | $ | 2,635 | $ | 81,668 | $ | 5,800 | $ | 12,408 | $ | - | $ | 1,127 | $ | (100,623 | ) | $ | 3,287 | $ | - | $ | 3,015 | |||||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-8
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
In thousands (except for share and per share data)
| Year ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| USD | ||||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net loss | $ | (6,571 | ) | $ | (6,433 | ) | $ | (8,393 | ) | |||
| Adjustments to reconcile loss to net cash used: | ||||||||||||
| Depreciation of property, plant and equipment | 14 | 19 | 18 | |||||||||
| Share-based payment | 535 | 192 | 309 | |||||||||
| Decrease in severance pay, net | - | - | (152 | ) | ||||||||
| Changes in fair value of warrants liability exercisable into shares | - | (72 | ) | (232 | ) | |||||||
Changes in fair value of short-term investment (previously long-term) | 644 | 5 | - | |||||||||
| Gain from sale of investment in previously consolidated subsidiaries (a) | - | (769 | ) | - | ||||||||
| Exchange differences on balances of cash and cash equivalents | 89 | 83 | 82 | |||||||||
| 1,282 | (542 | ) | 25 | |||||||||
| Working capital adjustments: | ||||||||||||
| Increase in accounts receivable, prepaid expenses and lease deposit | (853 | ) | (2,907 | ) | (1,397 | ) | ||||||
| Decrease in trade payable | 644 | 293 | 816 | |||||||||
| Increase (decrease) in deferred revenues | 1,218 | (289 | ) | 335 | ||||||||
| Increase (decrease) in other accounts payable | 125 | 906 | (100 | ) | ||||||||
| 1,134 | (1,997 | ) | (346 | ) | ||||||||
| Net cash used in operating activities | $ | (4,155 | ) | $ | (8,972 | ) | $ | (8,714 | ) | |||
The accompanying notes are an integral part of the consolidated financial statements.
F-9
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
In thousands (except for share and per share data)
| Year ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| USD | ||||||||||||
| Cash flows from investing activities: | ||||||||||||
| Purchase of property, plant and equipment | $ | (33 | ) | $ | (7 | ) | $ | (10 | ) | |||
| Proceeds from sale of investments in previously consolidated subsidiaries (a) | - | (22 | ) | - | ||||||||
| Net cash used in investing activities | (33 | ) | (29 | ) | (10 | ) | ||||||
| Cash flows from financing activities: | ||||||||||||
| Issuance of share capital and warrants, net of issuance expenses | 4,387 | 4,474 | - | |||||||||
| Net cash provided by financing activities | 4,387 | 4,474 | - | |||||||||
| Exchange differences on balances of cash and cash equivalents | (89 | ) | (83 | ) | (82 | ) | ||||||
| Increase (decrease) in cash and cash equivalents | 110 | (4,610 | ) | (8,806 | ) | |||||||
| Cash and cash equivalents at the beginning of the year | 3,505 | 8,115 | 16,921 | |||||||||
| Cash and cash equivalents at the end of the year | 3,615 | 3,505 | 8,115 | |||||||||
| Supplemental disclosure of cash flow information: | ||||||||||||
| Cash paid during the year for income taxes | 4 | 29 | 29 | |||||||||
| Cash received during the year for interest | $ | 51 | $ | 69 | $ | 89 | ||||||
| Year ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| USD | ||||||||||||
| (a) Proceeds from sale of investments in previously consolidated subsidiary: | ||||||||||||
| The subsidiaries’ assets and liabilities at date of sale: | ||||||||||||
| Working capital (excluding cash and cash equivalents) | $ | - | $ | (53 | ) | $ | - | |||||
| Treasury shares deduction, net | - | 132 | - | |||||||||
| Non-controlling interests | - | 52 | - | |||||||||
| Gain (loss) from sale of subsidiaries | - | 769 | - | |||||||||
| Long term investments | - | (922 | ) | - | ||||||||
| $ | - | $ | (22 | ) | $ | - | ||||||
| *) | Represent an amount lower than USD 1. |
The accompanying notes are an integral part of the consolidated financial statements.
F-10
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 1:- GENERAL
| a. | Company description: |
Can-Fite Biopharma Ltd. (the “Company”) was incorporated and started to operate in September 1994 as a private Israeli company. Can-Fite is a clinical-stage biopharmaceutical company focused on developing orally bioavailable small molecule therapeutic products for the treatment of autoimmune-inflammatory, oncological and sexual dysfunction indications. Its platform technology utilizes the Gi protein associated A3AR as a therapeutic target. A3AR is highly expressed in inflammatory and cancer cells, and not significantly expressed in normal cells, suggesting that the receptor could be a unique target for pharmacological intervention. The Company’s pipeline of drug candidates are synthetic, highly specific agonists and allosteric modulators, or ligands or molecules that initiate molecular events when binding with target proteins, targeting the A3AR.
The Company’s ordinary shares have been publicly traded on the Tel-Aviv Stock Exchange since October 2005 under the symbol “CFBI” and the Company’s American Depositary Shares (“ADSs”) began public trading on the over the counter market in the U.S. in October 2012 and since November 2013 the Company’s ADSs have been publicly traded on the NYSE American under the symbol “CANF”.
| b. | The Company owned 82% of a U.S. based subsidiary, Ophthalix, Inc. which developed the CF101 drug for treatment of ophthalmic indications under license from the Company. The license to develop this drug was transferred from the Company to Ophthalix, Inc. in the context of an ophthalmic activity spinoff transaction. Ophthalix, Inc. was traded in the over the counter market in the U.S. under the symbol “OPLI”. |
On May 21, 2017, OphthaliX and a wholly-owned private Israeli subsidiary of OphthaliX, Bufiduck Ltd. (the “Merger Sub”), and Wize Pharma Ltd. (“Wize”), an Israeli company formerly listed on the Tel Aviv Stock Exchange currently focused on the treatment of ophthalmic disorders, including dry eye syndrome, entered into an Agreement and Plan of Merger (the “Merger Agreement”), providing for the merger of the Merger Sub with and into Wize, with Wize becoming a wholly-owned subsidiary of OphthaliX and the surviving corporation of the merger (the “Merger”). On November 16, 2017, the Merger was completed. As a result of the Merger, the Company’s ownership of OphthaliX, immediately post-Merger, became approximately 8% of the outstanding shares of common stock. In addition, immediately prior to the Merger, OphthaliX sold on an “as is” basis to the Company all the ordinary shares of Eyefite in exchange for the irrevocable cancellation and waiver of all indebtedness owed by OphthaliX and Eyefite to the Company, including approximately USD 5,000 of deferred payments owed by OphthaliX and Eyefite to the Company and, as part of the purchase of Eyefite, the Company also assumed certain accrued milestone payments in the amount of USD 175 under a license agreement previously entered into with the NIH. In addition, that certain exclusive license of Piclidenoson granted to OphthaliX by the Company and a related services agreement was terminated. In connection with the Merger, OphthaliX was renamed Wize Pharma, Inc.
As a result of the Merger, the Company recorded a capital gain of USD 769.
F-11
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 1:- GENERAL (Cont.)
| c. | During the year ended December 31, 2018, the Company incurred net losses of USD 6,571 and it had negative cash flows from operating activities in the amount of USD 4,155. |
Furthermore, the Company intends to continue to finance its operating activities by raising capital and seeking collaborations with multinational companies in the industry. There are no assurances that the Company will be successful in obtaining an adequate level of financing needed for its long-term research and development activities.
If the Company will not have sufficient liquidity resources, the Company may not be able to continue the development of all of its products or may be required to implement a cost reduction and may be required to delay part of its development programs. The Company’s management and board of directors are of the opinion that its current financial resources will be sufficient to continue the development of the Company’s products for at least the next twelve months.
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES
| a. | Definitions: |
In these consolidated financial statements:
| The Company | - | Can-Fite Biopharma Ltd. |
| The Group | - | The Company and its subsidiary (as defined below) |
| Subsidiaries | - | Companies that are controlled by the Company (as defined in IAS 27 (2008)) and whose accounts are consolidated with those of the Company |
| Wize Pharma, Inc. | - | Wize Pharma, Inc. (formerly OphthaliX Inc.) |
| Eye-Fite | - | Eye-Fite Ltd (Can-Fite’s wholly owned subsidiary) |
| Related parties | - | As defined in IAS 24 |
| NIS | - | New Israeli Shekel |
| USD | - | U.S. dollar |
| € | - | European Union Euro |
| CAD | - | Canadian dollar |
| ADS | - | American Depositary Share (“ADS”). Each ADS represents 2 ordinary shares of the Company |
The following accounting policies have been applied consistently in the financial statements for all periods presented, unless otherwise stated.
F-12
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| b. | Basis of presentation of the financial statements: |
These financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board.
The Company’s financial statements have been prepared on a cost basis, except for financial assets and liabilities (including warrants) which are presented at fair value through statement of comprehensive loss.
The preparation of the financial statements requires management to make critical accounting estimates as well as exercise judgment in the process of adopting significant accounting policies. The matters which required the exercise of significant judgment and the use of estimates, which have a material effect on amounts recognized in the financial statements, are specified in Note 3.
| c. | Consolidated financial statements: |
The consolidated financial statements comprise the financial statements of companies that are controlled by the Company (i.e., subsidiaries). Control is achieved when the Company is exposed, or has the rights, to variable returns from its involvement with the investee and has the ability to affect those returns through its power over the investee. The effect of potential voting rights that are exercisable at the end of the reporting period is considered when assessing whether an entity has control. The consolidation of the financial statements commences on the date on which control is obtained and ends when such control ceases.
The financial statements of the Company and of the subsidiaries are prepared as of the same dates and periods. The consolidated financial statements are prepared using uniform accounting policies by all companies in the Group. Significant intragroup balances and transactions and gains or losses resulting from intragroup transactions are eliminated in full in the consolidated financial statements.
Non-controlling interests in subsidiaries represent the non-controlling shareholders’ share of the total comprehensive loss of the subsidiaries and their share of the net assets. The non-controlling interests are presented in equity separately from the equity attributable to the equity holders of the Company. Losses are attributed to non-controlling interests even if they result in a negative balance of non-controlling interests in the consolidated statement of financial position.
Upon the disposal of a subsidiary resulting in loss of control, the Company:
| - | derecognizes the subsidiary’s assets (including goodwill) and liabilities. |
| - | derecognizes the carrying amount of non-controlling interests. |
| - | derecognizes the adjustments arising from translating financial statements carried to equity. |
| - | recognizes the fair value of the consideration received. |
| - | recognizes the fair value of any remaining investment. |
F-13
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| - | reclassifies the components previously recognized in other comprehensive income (loss) on the same basis as would be required if the subsidiary had directly disposed of the related assets or liabilities. |
| - | recognizes any resulting difference (surplus or deficit) as gain or loss. |
| d. | Functional currency, presentation currency and foreign currency: |
| 1. | Functional currency and presentation currency: |
From the Company’s inception through January 1, 2018, the Company’s functional and presentation currency was the NIS. Management conducted a review of the functional currency of the Company and decided to change its functional and presentation currency to the USD from the NIS effective January 1, 2018. These changes were based on an assessment by Company management that the USD is the primary currency of the economic environment in which the Company operates.
In determining the appropriate functional currency to be used, the Company followed the guidance in International Accounting Standard 21 - The Effects of Changes in Foreign Exchange Rates (“IAS 21”), which states that factors relating to sales, costs and expenses, financing activities and cash flows, as well as other potential factors, should be considered. In this regard, the Company is incurring and expects to continue to incur a majority of its expenses in USD as a result of its expanded clinical trials including Phase 3 trials. These changes, as well as the fact that the majority of the Company’s available funds are in USD, the Company’s principal source of financing is the U.S. capital market, and all of the Company’s budgeting is conducted solely in U.S. dollars, led to the decision to make the change in functional currency as of January 1, 2018, as indicated above.
At the date of change of functional currency, the Company also changed the presentation currency of these financial statements to the USD. This change was retrospectively implemented. In accordance with IAS 21, since the Company’s presentation currency was different than its functional currency, results and financial position were translated using the following principles: (i) all assets and liabilities were translated using the current exchange rates, (ii) equity accounts were translated using the historical rates, and (iii) income and expenses for each statement of comprehensive income or separate income statement presented were translated at exchange rates at the dates of the transactions.
The Company also implements the guidance in IAS 21 regarding translating foreign currency financial statements of consolidated subsidiaries.
F-14
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| 2. | Transactions, assets and liabilities in foreign currency: |
Transactions denominated in foreign currency are recorded upon initial recognition at the exchange rate at the date of the transaction.
After initial recognition, monetary assets and liabilities denominated in foreign currency are translated at the end of each reporting period into the functional currency at the exchange rate at that date. Exchange rate differences are recognized in statement of comprehensive loss.
Non-monetary assets and liabilities measured at cost in foreign currency are translated at the exchange rate at the date of the transaction.
Non-monetary assets and liabilities denominated in foreign currency and measured at fair value are translated into the functional currency using the exchange rate prevailing at the date when the fair value was determined.
| e. | Cash equivalents: |
Cash equivalents are considered as highly liquid investments, including unrestricted short-term bank deposits with an original maturity of three months or less from the investment date.
| f. | Account receivables and prepaid expenses: |
Prepaid expenses are composed mainly from active pharmaceutical ingredients and clinical trial drug-kits which are expensed based on the percentage of completion method of the related clinical trials.
| g. | Property, plant and equipment: |
Property, plant and equipment are measured at cost, including directly attributable costs, less accumulated depreciation, accumulated impairment losses and excluding day-to-day servicing expenses.
Depreciation is calculated on a straight-line basis over the useful life of the assets at annual rates as follows:
| % | Mainly % |
||||
| Laboratory equipment and Leasehold improvements | 10 | ||||
| Computers, office furniture and equipment | 6 - 33 | 33 | |||
Leasehold improvements are depreciated on a straight-line basis over the shorter of the lease term (including extension option held by the Company and intended to be exercised) and the expected life of the improvement.
The useful life, depreciation method and residual value of an asset are reviewed at least each year-end and any changes are accounted for prospectively as a change in accounting estimates. Depreciation of an asset ceases at the earlier of the date that the asset is classified as held for sale and the date that the asset is derecognized.
F-15
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| h. | Revenue recognition: |
The Company generates revenues from distribution agreements. Such revenues comprises of upfront license fees, milestone payments and potential royalty payments.
The Company identified four components in the agreements: (i) performing the research and development services through regulatory approval; (ii) exclusive license to distribute the product; (iii) participation in joint steering committee; and, (iv) royalties resulting from future sales of the product.
As described in Note 4 regarding the initial adoption of IFRS 15, “Revenue from Contracts with Customers” (“the Standard”), the Company elected to adopt the provisions of the Standard using the modified retrospective method with the application of certain practical expedients and without restatement of comparative data.
The accounting policy for revenue recognition applied until December 31, 2017, is as follows:
The Company recognizes revenue in accordance with IAS 18, “Revenue” pursuant to which each required deliverable is evaluated to determine whether it qualifies as a separate unit of accounting based on whether the deliverable has “stand-alone value” to the customer. The arrangement’s consideration that is fixed or determinable is then allocated to each separate unit of accounting based on the relative selling price of each deliverable which is based on the Estimated Selling Price (“ESP”).
Components (i) – (iii) were analyzed as one unit of accounting. Consequently, revenue from these components is recorded based on the term of the research and development services (which is the last deliverable in the arrangement).
Contingent payments related to milestones were recognized immediately upon satisfaction of the milestone and contingent payments related to royalties were recognized in the period that the related sales have occurred.
Revenues from royalties will be recognized as they accrue in accordance with the terms of the relevant agreement.
The accounting policy for revenue recognition applied commencing from January 1, 2018, is as follows:
Revenue recognition:
Revenue from contracts with customers is recognized when the control over the goods or services is transferred to the customer. The transaction price is the amount of the consideration that is expected to be received based on the contract terms, excluding amounts collected on behalf of third parties (such as taxes).
Revenue from contracts with strategic partners:
Revenue from contracts with strategic partners are recognized over time as the Company satisfies the performance obligations. The Company usually accepts long-term upfront payment from its strategic partners. Contract liabilities for those upfront payments are recognizes as revenue over time.
Variable consideration:
The Company evaluates the individual contracts to determine the estimated variable consideration and related constraint.
Significant financing component:
The Company receives long-term advances. The transaction price for such contracts is discounted, using the rate that would be reflected in a separate financing transaction between the Company and its advances at contract inception, to take into consideration the significant financing component. Contract liabilities due to the upfront payments are recognized as revenue when the Group performs under the contract. See also Note 4.
F-16
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| i. | Research and development expenditures: |
Research expenditures are recognized in the statement of comprehensive loss when incurred.
| j. | Impairment of non-financial assets: |
The Company evaluates the need to record an impairment of the carrying amount of non financial assets whenever events or changes in circumstances indicate that the carrying amount is not recoverable. If the carrying amount of property, plant and equipment exceeds their recoverable amount, the property, plant and equipment are reduced to their recoverable amount. The recoverable amount is the higher of fair value less costs of sale and value in use. In measuring value in use, the expected future cash flows are discounted using a pre-tax discount rate that reflects the risks specific to the asset. The recoverable amount of an asset that does not generate independent cash flows is determined for the cash-generating unit to which the asset belongs. Impairment losses are recognized in profit or loss. As of December 31, 2018 and 2017, no impairment indicators have been identified.
| k. | Financial instruments: |
As described in Note 4 regarding the initial adoption of IFRS 9, “Financial Instruments” (“the Standard”), the Company elected to adopt the provisions of the Standard retrospectively without restatement of comparative data.
The accounting policy for financial instruments applied until December 31, 2017, is as follows:
| 1. | Financial assets: |
Financial assets within the scope of IAS 39 are initially recognized at fair value plus directly attributable transaction costs, except for financial assets measured at fair value through profit or loss in respect of which transaction costs are recorded in profit or loss.
After initial recognition, the accounting treatment of financial assets is based on their classification as follows:
| a) | Financial assets at fair value through profit or loss: |
This category includes financial assets held for trading and financial assets designated upon initial recognition as at fair value through profit or loss.
| b) | Receivables: |
Receivables are investments with fixed or determinable payments that are not quoted in an active market. Short-term borrowings are measured based on their terms, normally at face value.
| c) | Available-for-sale financial assets: |
Available-for-sale financial assets are (non-derivative) financial assets that are designated as available for sale or are not classified in any of the three preceding categories. After initial recognition, available-for-sale financial assets are measured at fair value.
| 2. | Financial liabilities: |
Financial liabilities are initially recognized at fair value.
F-17
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
After initial recognition, the accounting treatment of financial liabilities is based on their classification as follows:
| a) | Financial liabilities at amortized cost: |
After initial recognition, loans and other liabilities are measured based on their terms at amortized cost less directly attributable transaction costs using the effective interest method.
| 3. | Issue of a unit of securities: |
The issue of a unit of securities involves the allocation of the proceeds received (before issue expenses) to the components of the securities issued in the unit based on the following order: financial derivatives and other financial instruments measured at fair value in each period. Then fair value is determined for financial liabilities and compound instruments that are presented at amortized cost. The consideration allocated to the equity instruments is determined as the residual value. The issuance costs are allocated to each component based on the amounts allocated to each component in the unit.
| 4. | Derecognition of financial instruments: |
| a) | Financial assets: |
A financial asset is derecognized when the contractual rights to the cash flows from the financial asset expire or the Company has transferred its contractual rights to receive cash flows from the financial asset or assumes an obligation to pay the cash flows in full without material delay to a third party and has transferred substantially all the risks and rewards of the asset, or has neither transferred nor retained substantially all the risks and rewards of the asset, but has transferred control of the asset.
If the Company transfers its rights to receive cash flows from an asset and neither transfers nor retains substantially all the risks and rewards of the asset nor transfers control of the asset, a new asset is recognized to the extent of the Company’s continuing involvement in the asset. When continuing involvement takes the form of guaranteeing the transferred asset, the extent of the continuing involvement is the lower of the original carrying amount of the asset and the maximum amount of consideration received that the Company could be required to repay.
| b) | Financial liabilities: |
A financial liability is derecognized when it is extinguished, that is when the obligation is discharged, realized, cancelled or expires. A financial liability is extinguished when the debtor (i.e., the Group) discharges the liability by paying in cash, other financial assets, goods or services or shares, or is legally released from the liability.
F-18
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
The accounting policy for financial instruments applied commencing from January 1, 2018, is as follows:
| 1. | Financial assets: |
Financial assets are measured upon initial recognition at fair value plus transaction costs that are directly attributable to the acquisition of the financial assets, except for financial assets measured at fair value through profit or loss in respect of which transaction costs are recorded in profit or loss.
The Company classifies and measures debt instruments in the financial statements based on the following criteria:
| - | The Company’s business model for managing financial assets; and |
| - | The contractual cash flow terms of the financial asset. |
Equity instruments and other financial assets held for trading:
Investments in equity instruments do not meet the above criteria and accordingly are measured at fair value through profit or loss.
Impairment of financial assets:
The Company reviews at the end of each reporting period the provision for loss of financial debt instruments which are not measured at fair value through profit or loss. The Company distinguishes between two types of provision for losses:
| a. | Debt instruments whose credit quality has not significantly deteriorated since their initial recognition date or whose credit risk is low—the provision for loss that will be recognized in respect of this debt instrument will take into account expected credit losses within 12 months from the reporting date; or |
| b. | Debt instruments whose credit quality has significantly deteriorated since their initial recognition date or whose credit risk is not low—the provision for loss that will be recognized will take into account expected credit losses over the instrument’s remaining term. |
The Company has financial assets bearing short-term credit such as trade receivables in respect of which it is required to adopt the relief prescribed in the model i.e., the Company will measure the provision for loss in an amount which is equivalent to the expected credit losses.
An impairment loss of debt instruments measured at amortized cost is carried to profit or loss against a provision whereas an impairment loss of debt instruments measured at fair value through other comprehensive income will be carried against a capital reserve and will not reduce the carrying amount of the financial asset in the statement of financial position.
Derecognition of financial assets:
A financial asset is derecognized only when the following criteria are met:
| a. | The contractual rights to the cash flows from the financial asset expire; or |
| b. | The Company has transferred substantially all the risks and rewards deriving from the contractual rights to receive cash flows from the financial asset or has neither transferred nor retained substantially all the risks and rewards of the asset, but has transferred control of the asset. |
| c. | The Company has retained its contractual rights to receive cash flows from the financial asset but has assumed a contractual obligation to pay the cash flows in full without material delay to a third party. |
F-19
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| 2. | Financial liabilities: |
Financial liabilities are initially recognized at fair value less transaction costs that are directly attributable to the issue of the financial liability, excluding financial liabilities measured at fair value through profit or loss whose transaction costs are carried to profit or loss.
After initial recognition, the Company measures all financial liabilities at amortized cost.
Derecognition of financial liabilities:
A financial liability is derecognized only when it is extinguished, that is when the obligation is discharged, cancelled or expires. A financial liability is extinguished when the debtor discharges the liability by paying in cash, other financial assets, goods or services; or is legally released from the liability.
| 3. | Issue of a unit of securities: |
The issue of a unit of securities involves the allocation of the proceeds received (before issue expenses) to the securities issued in the unit based on the following order: financial derivatives and other financial instruments measured at fair value in each period. Then fair value is determined for financial liabilities that are measured at amortized cost. The proceeds allocated to equity instruments are determined to be the residual amount. Issue costs are allocated to each component pro rata to the amounts determined for each component in the unit.
| l. | Fair value measurement: |
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
Fair value measurement is based on the assumption that the transaction will take place in the asset’s or the liability’s principal market, or in the absence of a principal market, in the most advantageous market.
The fair value of an asset or a liability is measured using the assumptions that market participants would use when pricing the asset or liability, assuming that market participants act in their economic best interest.
Fair value measurement of a non-financial asset takes into account a market participant’s ability to generate economic benefits by using the asset in its highest and best use or by selling it to another market participant that would use the asset in its highest and best use.
The Group uses valuation techniques that are appropriate in the circumstances and for which sufficient data are available to measure fair value, maximizing the use of relevant observable inputs and minimizing the use of unobservable inputs.
All assets and liabilities measured at fair value or for which fair value is disclosed are categorized into levels within the fair value hierarchy based on the lowest level input that is significant to the entire fair value measurement. See also Note 9.
| m. | Treasury shares: |
Company shares held by OphthaliX are recognized at cost, and as a deduction from equity. Any gain or loss arising from a purchase, sale, issuance or cancellation of treasury shares is recognized directly in equity. As of December 31, 2018, the Company has no treasury shares. Please refer to note 1.b.
| n. | Provisions: |
A provision in accordance with IAS 37 is recognized when the Group has a present obligation (legal or constructive) as a result of a past event, it is probable that an outflow of resources embodying economic benefits will be required to settle the obligation and a reliable estimate can be made of the amount of the obligation. If the Group expects part or all of the expense to be reimbursed to the Company, such as in an insurance contract, the reimbursement is recognized as a separate asset only when it is virtually certain that it will be received by the Company. The expense is recognized in the income statement net of the reimbursed amount.
F-20
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
Legal claims:
A provision for claims is recognized when the Group has a present legal or constructive obligation as a result of a past event, it is more likely than not that an outflow of resources embodying economic benefits will be required by the Group to settle the obligation and a reliable estimate can be made of the amount of the obligation. No provisions pursuant to IAS 37 have been identified.
| o. | Employee benefit liabilities: |
The Company’s liability for severance pay is pursuant to Section 14 of the Severance Compensation Act, 1963 (“Section 14”), pursuant to which all the Company’s employees are included under Section 14, and are entitled only to monthly deposits, at a rate of 8.33% of their monthly salary, made in the employee’s name with insurance companies. Under Israeli employment law, payments in accordance with Section 14 release the Company from any future severance payments in respect of those employees. The fund is made available to the employee at the time the employer-employee relationship is terminated, regardless of cause of termination. The severance pay liabilities and deposits under Section 14 are not reflected in the consolidated balance sheets as the severance pay risks have been irrevocably transferred to the severance funds.
| p. | Share-based payment transactions: |
The Company’s employees and other service providers are entitled to remuneration in the form of equity-settled share-based payment transactions. The cost of equity-settled transactions with employees is measured at the fair value of the equity instruments granted at grant date. The fair value is determined using the binomial option pricing model.
As for other service providers, the cost of the transactions is measured at the fair value of the goods or services received as consideration for equity instruments. In cases where the fair value of the goods or services received as consideration of equity instruments cannot be measured, they are measured by reference to the fair value of the equity instruments granted using binomial option pricing model.
The cost of equity-settled transactions is recognized in statement of comprehensive loss, together with a corresponding increase in equity, during the period which the performance and/or service conditions are to be satisfied, ending on the date on which the relevant employees become fully entitled to the award (the “Vesting Period”).
The cumulative expense recognized for equity-settled transactions at the end of each reporting period until the vesting date reflects the extent to which the Vesting Period has expired and the Group’s best estimate of the number of equity instruments that will ultimately vest.
F-21
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| q. | Taxes on income: |
As it is not likely that taxable income will be generated in the foreseeable future, deferred tax assets due to accumulated losses is not recognized in the Group’s financial statements.
| r. | Loss per share: |
Losses per share are calculated by dividing the net loss attributable to equity holders of the Company by the weighted number of ordinary shares outstanding during the period. Potential ordinary shares (warrants and unlisted options) are only included in the computation of diluted loss per share when their conversion increases loss per share from continuing operations. Potential ordinary shares that are converted during the period are included in diluted loss per share only until the conversion date and from that date in basic loss per share.
NOTE 3:- SIGNIFICANT ACCOUNTING JUDGMENTS, ESTIMATES AND ASSUPMTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS
In the process of applying the significant accounting policies, the Group has made the following judgments which have the most significant effect on the amounts recognized in the financial statements:
| - | Estimates and assumptions: |
The preparation of the financial statements requires management to make estimates and assumptions that have an effect on the application of the accounting policies and on the reported amounts of assets, liabilities and expenses. Changes in accounting estimates are reported in the period of the changes in estimates.
The key assumptions made in the financial statements concerning uncertainties at the end of the reporting period and the critical estimates computed by the Group that may result in a material adjustment to the carrying amounts of assets and liabilities within the next financial year are discussed below.
| - | Determining the fair value of share-based payment transactions: |
The fair value of share-based payment transactions is determined using an acceptable option-pricing model. The model includes data as to the share price and exercise price, and assumptions regarding expected volatility, expected life, expected dividend and risk-free interest rate.
| - | Legal claims: |
In estimating the likelihood of outcome of legal claims filed against the Company and its subsidiaries, the companies rely on the opinion of their legal counsel. These estimates are based on the legal counsel’s best professional judgment, taking into account the stage of proceedings and legal precedents in respect of the different issues. Since the outcome of the claims will be determined in courts, the results could differ from these estimates.
F-22
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 3:- SIGNIFICANT ACCOUNTING JUDGMENTS, ESTIMATES AND ASSUPMTIONS USED IN THE PREPARATION OF THE FINANCIAL STATEMENTS (Cont.)
| - | Deferred tax assets: |
Deferred tax assets are recognized for unused carryforward tax losses and deductible temporary differences to the extent that it is probable that taxable profit will be available against which the losses can be utilized. Significant management judgment is required to determine the amount of deferred tax assets that can be recognized, based upon the timing and level of future taxable profits, its source and the tax planning strategy.
NOTE 4:- DISCLOSURE OF NEW IFRS IN THE PERIOD
| a. | IFRS 15 – Revenues from contracts with customers: |
IFRS 15 supersedes IAS 11 Construction Contracts, IAS 18 Revenue and related Interpretations and it applies to all revenue arising from contracts with customers, unless those contracts are in the scope of other standards. The new standard replaces IAS 18, “Revenue”, IAS 11, “Construction Contracts”, IFRIC 13, “Customer Loyalty Programs”, IFRIC 15, “Agreements for the Construction of Real Estate”, IFRIC 18, “Transfers of Assets from Customers” and SIC-31, “Revenue - Barter Transactions Involving Advertising Services”. The new standard establishes a five-step model to account for revenue arising from contracts with customers.
Step 1: Identify the contract with a customer, including reference to contract combination and accounting for contract modifications.
Step 2: Identify the separate performance obligations in the contract
Step 3: Determine the transaction price, including reference to variable consideration, financing components that are significant to the contract, non-cash consideration and any consideration payable to the customer.
Step 4: Allocate the transaction price to the separate performance obligations on a relative stand-alone selling price basis using observable information, if it is available, or using estimates and assessments.
Step 5: Recognize revenue when a performance obligation is satisfied, either at a point in time or over time.
Under IFRS 15, revenue is recognized at an amount that reflects the consideration to which an entity expects to be entitled in exchange for transferring goods or services to a customer. The standard requires entities to exercise judgment, taking into consideration all of the relevant facts and circumstances when applying each step of the model to contracts with their customers. The standard also specifies the accounting for the incremental costs of obtaining a contract and the costs directly related to fulfilling a contract.
Revenue from contracts with customers is recognized when control of the goods or services are transferred to the customer at an amount that reflects the consideration to which the Group expects to be entitled in exchange for those goods or services.
The new standard has been applied for the first time in these financial statements. The Company elected to adopt the provisions of the new standard using the modified retrospective method and elected to apply that method to all contracts that were not completed at the date of initial application and without restatement of comparative data. The Company recognizes any difference between the previous carrying amount and the carrying amount on the date of initial application of the new standard as an adjustment to the opening balance of retained earnings.
The effect of the initial application of the new standard on the Company’s financial statements is as follows:
Advances payment - in certain service contracts, the Company receives advances from before the services are rendered. Before the application of the provisions of the new standard, the Company recognized revenue based on the consideration received and did not accrue interest on the advances. According to the new standard, when long-term advances (exceeding one year) are received for a service which the Company is to provide in the future, the Company accrues interest and recognizes finance expense on the advances over the expected period of the contract, provided that the contract contains a significant financing component, as defined in the new standard. As the advances are recognized in revenue, the Company also recognizes the accrued interest as part of revenue from services. As a result of the application of the new standard, the finance expenses included in the Company’s financial statements are higher in the period from the date of receipt of the advance and the date of performance of the service. Also, the revenue recognized on the date of performance of the service is higher.
The table discloses IFRS 15 impact as of January 1, 2018, as of December 31, 2018 and for the year then ended:
F-23
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 4:- DISCLOSURE OF NEW IFRS IN THE PERIOD (Cont.)
| As of January 1, 2018 | ||||||||||||
| As reported (IFRS 15) | Adjustments | IAS 18 (excluding impact of IFRS 15) | ||||||||||
| Current liabilities | ||||||||||||
| Deferred revenues | $ | 280 | $ | 50 | $ | 330 | ||||||
| Non - current liabilities | ||||||||||||
| Deferred revenues | $ | 1,246 | $ | (400 | ) | $ | 846 | |||||
| Equity attributable to equity holders of the Company | ||||||||||||
| Accumulated deficit | $ | (94,052 | ) | $ | 350 | $ | (93,702 | ) | ||||
| As of December 31, 2018 | ||||||||||||
| As reported (IFRS 15) | Adjustments | IAS 18 (excluding impact of IFRS 15) | ||||||||||
| Current liabilities | ||||||||||||
| Deferred revenues | $ | 926 | $ | (26 | ) | $ | 900 | |||||
| Non - current liabilities | ||||||||||||
| Deferred revenues | $ | 1,818 | $ | (588 | ) | $ | 1,230 | |||||
| Equity attributable to equity holders of the Company | ||||||||||||
| Accumulated deficit | $ | (94,052 | ) | $ | 350 | $ | (93,702 | ) | ||||
| Year ended December 31, 2018 | ||||||||||||
| As reported (IFRS 15) | Adjustments | IAS 18 (excluding impact of IFRS 15) | ||||||||||
| Revenues | $ | 3,820 | $ | (201 | ) | $ | 3,619 | |||||
| Operating expenses | (9,234 | ) | - | (9,234 | ) | |||||||
| Operating loss | (5,414 | ) | (201 | ) | (5,615 | ) | ||||||
| Financial expense, net | (1,153 | ) | 426 | (727 | ) | |||||||
| Loss before taxes on income | $ | (6,567 | ) | $ | 225 | $ | (6,342 | ) | ||||
| Taxes on income | (4 | ) | - | (4 | ) | |||||||
| Net loss | (6,571 | ) | (6,346 | ) | ||||||||
| . | ||||||||||||
| Basic and diluted net loss per share | $ | 0.08 | $ | - | $ | 0.08 | ||||||
In implementing IFRS 15, the Company considered the following:
| (1) | Variable consideration: |
Some contracts with customers provide a right of return, trade discounts or volume rebates. Currently, the Company recognizes revenue from achieving milestones, net of returns and allowances, trade discounts and volume rebates. If revenue cannot be reliably measured, the Company defers revenue recognition until the uncertainty is resolved. Such provisions give rise to variable consideration under IFRS 15, which will be required to be estimated at contract inception.
F-24
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 4:- DISCLOSURE OF NEW IFRS IN THE PERIOD (Cont.)
IFRS 15 requires that the variable consideration be estimated conservatively to prevent over-recognition of revenue.
The Company continues to assess individual contracts to determine the estimated variable consideration and related constraint. There is no impact of IFRS 15 on the financial statements.
| (2) | Significant financing component: |
The Company receives long-term advances. The transaction price for such contracts is discounted, using the rate that would be reflected in a separate financing transaction between the Company and its advances at contract inception, to take into consideration the significant financing component. Contract liabilities due to the upfront payments are recognized as revenue when the Group performs under the contract.
| (3) | Satisfaction of performance obligation: |
Revenue from contracts with strategic partners are recognized over time as the Company satisfies the performance obligations. The Company usually accepts long-term upfront payment from its strategic partners. Contract liabilities for those upfront payments and recognizes as revenue over time.
IFRS 9 - Financial Instruments:
In July 2014, the IASB issued the final and complete version of IFRS 9, “Financial Instruments”, which replaces IAS 39, “Financial Instruments: Recognition and Measurement”. The new standard mainly focuses on the classification and measurement of financial assets and it applies to all assets within the scope of IAS 39.
The new standard has been applied for the first time in these financial statements retrospectively without restatement of comparative data.
The effect of the initial adoption of the new standard on the Company’s financial statements is as follows:
Under IFRS 9, the classification of financial assets at initial recognition depends on the financial asset’s contractual cash flow characteristics and the Group’s business model for managing them. The following is the relevant accounting policy to financial instruments of the Company:
Financial assets are classified, at initial recognition, as subsequently measured at amortized cost, fair value through other comprehensive income (OCI), and fair value through profit or loss.
The adoption of IFRS 9 has changed the Company’s accounting for impairment losses for financial assets by replacing IAS 39’s incurred loss approach with a forward-looking expected credit loss (ECL) approach. IFRS 9 requires the Company to record an allowance for ECLs for all loans and other debt financial assets not held at FVPL.
F-25
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 4:- DISCLOSURE OF NEW IFRS IN THE PERIOD (Cont.)
ECLs are based on the difference between the contractual cash flows due in accordance with the contract and all the cash flows that the Company expects to receive. For other debt financial assets (i.e., debt securities at fair value through other comprehensive income), the ECL is based on the 12-month ECL. The 12-month ECL is the portion of lifetime ECLs that result from default events on a financial instrument that are possible within 12 months after the reporting date.
DISCLOSURE OF NEW STANDARDS IN THE PERIOD PRIOR TO THEIR ADOPTION
IFRS 16, “Leases”:
In January 2016, the IASB issued IFRS 16, “Leases”. According to the new Standard, a lease is a contract, or part of a contract, that conveys the right to use an asset for a period of time in exchange for consideration.
The effects of the adoption of the new standard are as follows:
| ● | According to the new standard, lessees are required to recognize all leases in the statement of financial position (excluding certain exceptions, see below). Lessees will recognize a liability for lease payments with a corresponding right-of-use asset, similar to the accounting treatment for finance leases under the existing standard, IAS 17, “Leases”. Lessees will also recognize interest expense and depreciation expense separately. |
| ● | Variable lease payments that are not dependent on changes in the Consumer Price Index (“CPI”) or interest rates, but are based on performance or use are recognized as an expense by the lessees as incurred and recognized as income by the lessors as earned. |
| ● | In the event of change in variable lease payments that are CPI-linked, lessees are required to remeasure the lease liability and record the effect of the remeasurement as an adjustment to the carrying amount of the right-of-use asset. |
| ● | The accounting treatment by lessors remains substantially unchanged from the existing standard, namely classification of a lease as a finance lease or an operating lease. |
| ● | The new standard includes two exceptions which allow lessees to account for leases based on the existing accounting treatment for operating leases - leases for which the underlying asset is of low financial value and short-term leases (up to one year). |
The new standard is effective for annual periods beginning on or after January 1, 2019.
The new standard permits lessees to use one of the following approaches:
1. Full retrospective approach - according to this approach, a right-of-use asset and the corresponding liability will be presented in the statement of financial position as if they had always been measured according to the provisions of the new standard. Accordingly, the effect of the adoption of the new standard at the beginning of the earliest period presented will be recorded in equity. Also, the Company will restate the comparative data in its financial statements. Under this approach, the balance of the liability as of the date of initial application of the new standard will be calculated using the interest rate implicit in the lease, unless this rate cannot be easily determined in which case the lessee’s incremental borrowing rate of interest on the commencement date of the lease will be used.
2. Modified retrospective approach - this approach does not require restatement of comparative data. The balance of the liability as of the date of initial application of the new standard will be calculated using the lessee’s incremental borrowing rate of interest on the date of initial application of the new standard. As for the measurement of the right-of-use asset, the Company may choose, on a lease-by-lease basis, to apply one of the two following alternatives:
| ● | Recognize an asset in an amount equal to the lease liability, with certain adjustments. |
| ● | Recognize an asset as if the new standard had always been applied. |
Any difference arising on the date of first-time recorded in equity.
The Company believes, based on an assessment of the impact of the adoption of the new standard, that its application is not expected to have a material effect on the financial statements.
F-26
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 5:- OTHER ACCOUNTS RECEIVABLE AND PREPAID EXPENSES
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| USD | ||||||||
| Government authorities | $ | 41 | $ | 66 | ||||
| Prepaid expenses and others | $ | 3,974 | $ | 3,093 | ||||
| $ | 4,015 | $ | 3,159 | |||||
NOTE 6:- SHORT-TERM INVESTMENT
The Company holds 356,803 shares of Wize Pharma Inc. as of December 31, 2018 and December 31, 2017 which as of such date represents 3.9% and 8.2% percent of Wize Pharma Inc’s outstanding shares, respectively. The shares are classified as financial asset as designated at fair value through profit or loss. As of December 31, 2017, the investment was classified under non-current assets.
NOTE 7:- PROPERTY, PLANT AND EQUIPMENT, NET
Balance as of December 31, 2018:
| Laboratory equipment | Computers, office furniture and equipment | Leasehold improvements | Total | |||||||||||||
| USD | ||||||||||||||||
| Cost: | ||||||||||||||||
| Balance at January 1, 2018 | $ | 22 | $ | 180 | $ | 6 | $ | 208 | ||||||||
| Purchases during the year | 17 | 10 | 6 | 33 | ||||||||||||
| Balance at December 31, 2018 | 39 | 190 | 12 | 241 | ||||||||||||
| Accumulated depreciation: | ||||||||||||||||
| Balance at January 1, 2018 | 14 | 161 | 5 | 180 | ||||||||||||
| Depreciation during the year | 4 | 9 | 1 | 14 | ||||||||||||
| Balance at December 31, 2018 | 18 | 170 | 6 | 194 | ||||||||||||
| Depreciated cost at December 31, 2018 | $ | 21 | $ | 20 | $ | 6 | $ | 47 | ||||||||
F-27
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 7:- PROPERTY, PLANT AND EQUIPMENT, NET (Cont.)
Balance as of December 31, 2017:
| Laboratory equipment | Computers, office furniture and equipment | Leasehold improvements | Total | |||||||||||||
| USD | ||||||||||||||||
| Cost: | ||||||||||||||||
| Balance at January 1, 2017 | $ | 22 | $ | 173 | $ | 6 | $ | 201 | ||||||||
| Purchases during the year | - | 7 | - | 7 | ||||||||||||
| Balance at December 31, 2017 | 22 | 180 | 6 | 208 | ||||||||||||
| Accumulated depreciation: | ||||||||||||||||
| Balance at January 1, 2017 | 10 | 146 | 5 | 161 | ||||||||||||
| Depreciation during the year | 4 | 15 | *-) | 19 | ||||||||||||
| Balance at December 31, 2017 | 14 | 161 | 5 | 180 | ||||||||||||
| Depreciated cost at December 31, 2017 | $ | 8 | $ | 19 | $ | 1 | $ | 28 | ||||||||
*) Represents an amount less than USD 1.
NOTE 8:- OTHER ACCOUNTS PAYABLE
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| USD | ||||||||
| Employees and payroll accruals | $ | 149 | $ | 225 | ||||
| Accrued expenses | 973 | 772 | ||||||
| $ | 1,122 | $ | 997 | |||||
NOTE 9:- FINANCIAL INSTRUMENTS
| a. | Financial assets: |
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| USD | ||||||||
| Financial assets at fair value through profit or loss (classified as Level 2 in the fair value hierarchy): | ||||||||
| Short-term investment | $ | 273 | $ | - | ||||
| Long-term investment | $ | - | $ | 917 | ||||
F-28
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 9:- FINANCIAL INSTRUMENTS (Cont.)
| b. | Financial liabilities, interest-bearing loans and borrowings: |
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| USD | ||||||||
| Other financial liabilities at amortized cost: | ||||||||
| Trade payable | $ | 1,071 | $ | 427 | ||||
| Other account payable | 1,122 | 997 | ||||||
| $ | 2,193 | $ | 1,424 | |||||
| c. | Financial risks factors: |
The Group’s activities expose it to foreign exchange risk. The Group’s comprehensive risk management plan focuses on activities that reduce to a minimum any possible adverse effects on the Group’s financial performance.
The Company’s management identifies and manages financial risks.
| d. | Foreign exchange risk: |
The Group is exposed to foreign exchange risk resulting from the exposure to different currencies, mainly the NIS. Foreign exchange risk arises on recognized assets and liabilities that are denominated in a foreign currency other than the functional currency.
The Group acts to reduce the foreign exchange risk by managing an adequate part of the available liquid sources in or linked to the NIS.
Foreign currency sensitivity analysis:
The following table demonstrates the sensitivity test to a reasonably possible change of NIS exchange rates, with all other variables held constant. A 10% strengthening of the NIS would have increased equity and the income statement by the amounts shown below. The Company’s exposure to foreign currency changes for all other currencies is immaterial.
| December 31, 2018 | December 31, 2017 | |||||||
| Linked to NIS | 32 | 11 | ||||||
| e. | Fair value: |
The carrying amount of cash and cash equivalents, Short-term investments ,trade payables and other accounts payable approximate their fair value.
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. To increase the comparability of fair value measures, the following hierarchy prioritizes the inputs to valuation methodologies used to measure fair value:
F-29
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 9:- FINANCIAL INSTRUMENTS (Cont.)
Level 1 - Valuations based on unadjusted quoted prices for identical assets and liabilities in active markets.
Level 2 - Valuations based on observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data.
Level 3 - Valuations based on unobservable inputs reflecting assumptions, consistent with reasonably available assumptions made by other market participants. These valuations require significant judgment.
The Company’s warrants exercisable into shares liability and the long term investment are classified as Level 1 in the fair value hierarchy, and measured at fair value on a recurring basis.
Fair value measurements using significant unobservable inputs (Level 1):
| USD | ||||
| Balance at December 31, 2016 | $ | 564 | ||
| Changes in values of warrants exercisable into shares liability | (564 | ) | ||
| Balance at December 31, 2017 | - | |||
| Changes in values of warrants exercisable into shares liability | - | |||
| Balance at December 31, 2018 | - | |||
F-30
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 9:- FINANCIAL INSTRUMENTS (Cont.)
Based on the Group’s policy, the Group generally mitigates the currency risk arising from recognized assets and recognized liabilities denominated in foreign currency other than the functional currency by maintaining part of the available liquid sources in deposits in foreign currency. Accordingly, the main currency exposures presented in the sensitivity tables are for those deposits.
NOTE 10:- CONTINGENT LIABILITIES AND COMMITMENTS
| a. | Liabilities to pay royalties: |
| 1. | According to the license agreement that the Company entered into with the NIH on January 29, 2003, the Company was committed to pay royalties until the expiration of the last patent licensed under the license agreement. The last patent under this agreement expired on June 29, 2015, and therefore except with respect to any amounts already accrued on the Company’s balance sheet, no future payments or royalties will be due. |
Following the Merger, the Company accrued USD 425 in other accounts payable with respect to the NIH.
| 2. | According to the patent license agreement that the Company entered into with Leiden University in the Netherlands on November 2, 2009, which is affiliated with the NIH, the Company was granted an exclusive license for the use of the patents of several compounds, including CF602 in certain territories. |
The Company is committed to pay royalties as follows:
| a) | A one-time concession commission of € 25; |
| b) | Annual royalties of € 10 until the clinical trials commence; |
| c) | 2%-3% of net sales (as defined in the agreement) received by the Company; |
| d) | Royalties in a total amount of up to € 850 based on certain progress milestones in the license stages of the products, which are the subject of the patent under the agreement, as follows: (i) € 50 upon initiation of Phase I studies; (ii) € 100 upon initiation of Phase II studies; (iii) € 200 upon initiation of Phase III studies; and (iv) € 500 upon marketing approval by any regulatory authority. |
| e) | If the agreement is sublicensed to another company, the Company will provide Leiden University royalties at a rate of 10%. A merger, consolidation or any other change in ownership will not be viewed as an assignment of the agreement as discussed in this paragraph. |
As of December 31 2018, no accrual is recorded with respect to Leiden University.
F-31
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 10:- CONTINGENT LIABILITIES AND COMMITMENTS (Cont.)
| b. | Commitments and license agreements: |
| 1. | In March 2015, the Company signed a distribution agreement with Cipher. As part of the distribution agreement, Cipher will distribute Can-Fite’s lead drug candidate, Piclidenoson for the treatment of psoriasis and rheumatoid arthritis in the Canadian market upon receipt of regulatory approvals. |
Under the terms of the agreement, Cipher made an upfront payment of USD 1,292 (CAD 1,650) to the Company in March 2015. In addition, the agreement provides that additional payments of up to CAD 2,000 will be received by the Company upon the achievement of certain milestones plus royalty payments of 16.5% of net sales of Piclidenoson in Canada.
The agreement further provides that the Company will deliver finished product to Cipher and that Cipher will reimburse the Company for the cost of manufacturing. Furthermore, under the distribution agreement, the Company shall be responsible for conducting product development activities including management of the clinical studies required in order to secure regulatory approvals, and shall use commercially reasonable efforts in conducting such activities. In addition the Company agreed to form a joint steering committee with Cipher which will oversee the progress of the clinical studies.
The Company identified four components in the agreement: (i) performing the research and development services through regulatory approval; (ii) an exclusive license to distribute the product in Canada; (iii) participation in joint steering committee; and,
(iv) royalties resulting from future sales of the product. Components (i) – (iii) were analyzed as one unit of accounting. Consequently, revenue from these components is recorded based on the term of the research and development services (which is the last deliverable in the arrangement). The Company estimates these services will be spread over a period of 24 quarters. Component (iv) was not accounted as part of the research and development services and will be recognized entirely upon the Company reaching the sales stage. The useful life, depreciation method and residual value of a liability are reviewed at least each year-end.
| 2. | In October 2016, the Company signed a distribution agreement with Chong Kun Dang Pharmaceuticals Corp. (“CKD”) for future sales in South Korea. As part of the distribution agreement, CKD will distribute Namodenoson for the treatment of liver cancer in the South Korean market upon receipt of regulatory approvals. |
Under the terms of the agreement, CKD made an upfront payment of USD 500 to the Company in December 2016 and in August 2017, the Company received a second milestone payment in the amount of USD 500 from CKD, which has licensed the exclusive right to distribute Namodenoson for the treatment of liver cancer in Korea upon receipt of regulatory approvals.
F-32
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 10:- CONTINGENT LIABILITIES AND COMMITMENTS (Cont.)
In addition, the agreement provides that additional payments of up to USD 2,500 will be received by the Company upon the achievement of certain milestones.
The agreement further provides that the Company will deliver finished product to CKD and that CKD will reimburse the Company for the cost of manufacturing for which the Company is entitled to a transfer price of the higher of the manufacturing cost plus 10% or 23% of net sales of Namodenoson in South Korea.
The Company identified four components in the agreement: (i) performing the research and development services through regulatory approval; (ii) an exclusive license to distribute the product in South Korea;(iii) participation in a joint steering committee; and, (iv) royalties resulting from future sales of the product. Components (i) – (iii) were analyzed as one unit of accounting. Consequently, revenue from these components is recorded based on the term of the research and development services (which is the last deliverable in the arrangement). The useful life, depreciation method and residual value of a liability are reviewed at least each year-end.
The Company estimates these services will spread over a period of 24 quarters. Component (iv) was not accounted as part of the research and development services and will be recognized entirely upon the Company reaching sales stage.
On February 25, 2019, the distribution agreement with CKD was amended (see Note 13).
| 3. | On December 22, 2008, the Company signed an agreement regarding the provision of a license for Piclodenoson with a South Korean pharmaceutical company, Kwang Dong Pharmaceutical Co. Ltd. (the “KD”). According to the license agreement, the Company granted the KD a license to use, develop and market its Piclodenoson for treating only rheumatoid arthritis only in the Republic of Korea. |
As of December 31, 2018, the Company estimates that such contingent payments are remote.
| 4. | On January 8, 2018, the Company entered into a Distribution and Supply Agreement with Gebro Holding GmBH (“Gebro”), granting Gebro the exclusive right to distribute Piclidenoson in Spain, Switzerland, Liechtenstein and Austria for the treatment of psoriasis and rheumatoid arthritis. |
Under the Distribution and Supply Agreement, the Company is entitled to €1,500 upon execution of the agreement plus milestone payments upon achieving certain clinical, launch and sales milestones, as follows: (i) €300 upon initiation of the ACRobat Phase III clinical trial for the treatment of rheumatoid arthritis and €300 upon the initiation of the COMFORT Phase III clinical trial for the treatment of psoriasis, (ii) between €750 and €1,600 following first delivery of commercial launch quantities of Piclidenson for either the treatment of rheumatoid arthritis or psoriasis, and (iii) between €300 and up to €4,025 upon meeting certain net sales. In addition, following regulatory approval, the Company shall be entitled to future royalties on net sales of Piclidenoson in the territories and payment for the manufacturing Piclidenoson. On January 25, 2018 the Company received a first payment of approximately USD 2,200 from Gebro and in August 2018 received approximately USD 350 upon reaching the first milstone.
F-33
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 10:- CONTINGENT LIABILITIES AND COMMITMENTS (Cont.)
| 5. | On August 6, 2018, the Company entered into a License, Collaboration and Distribution Agreement with CMS Medical Venture Investment Limited (“CMS Medical”) for the commercialization of Piclidenoson for the treatment of rheumatoid arthritis and psoriasis and Namodenoson for the treatment of advanced liver cancer and NAFLD/NASH in China (including Hong Kong, Macao and Taiwan). Under the License, Collaboration and Distribution Agreement, the Company received USD 2,000 upon execution of the agreement and is entitled to additional milestone payments upon achieving certain regulatory and sales milestones. In addition, following regulatory approval, the Company shall be entitled to future double digit royalties on net sales in the territories and payment for the manufacturing of Piclidenoson and Namodenoson. |
| 6. | Lease commitments: |
The Company lease one floor in one facility which expires on December 31, 2019. Lease payments are approximately USD 5 per month.
In addition, the Company leases motor vehicles through operating leases. The lease is for a period ending April 2021.
Future minimum lease commitments under non-cancelable operating leases as of December 31, 2018 are as follows:
| USD | ||||
| 2019 | $ | 80 | ||
| 2020-2021 | 10 | |||
| $ | 90 | |||
Motor lease expenses for the years ended December 31, 2017 and 2018 were approximately USD 58 and USD 56, respectively.
| c. | Class action: |
On June 29, 2015 the Company received a lawsuit requesting recognition of the lawsuit as a class action, naming the Company, its Chief Executive Officer and its directors as defendants. The lawsuit was filed with the District Court of Tel-Aviv.
The lawsuit alleged, among other things, that the Company misled the public with regard to disclosures concerning the efficacy of the Company’s drug candidate, Piclidenoson.
The claimant alleged that he suffered personal damages of over USD 20 (approximately NIS 73 based on the exchange rate reported by the Bank of Israel on December 31, 2018), while also claiming that the shareholders of the Company suffered damages of approximately USD 33,000 (approximately NIS 125,000 based on the exchange rate reported by the Bank of Israel on December 31, 2018). On July 18, 2017, the District Court of Tel-Aviv issued a ruling in which it denied the request to recognize the lawsuit as a class action and awarded the Company an amount of USD 14 (approximately NIS 50 thousands based on the exchange rate reported by the Bank of Israel on December 18, 2017) to pay the Company’s expenses in relation to such lawsuit.
F-34
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 10:- CONTINGENT LIABILITIES AND COMMITMENTS (Cont.)
On October 26, 2017, the claimant filed a petition with the Supreme Court appealing the District Court decision. On January 28, 2018, the Supreme Court issued a notice of procedures to be complied with by the relevant parties leading up to a formal hearing scheduled for December 5, 2018.
On December 5, 2018, the Supreme Court dismissed the appeal and as part of a compromise by the claimant not to pursue the appeal, the Supreme Court ordered the Company to return the aforementioned expenses to the claimant. Accordingly, this lawsuit has been finally dismissed and is no longer pending against the Company.
NOTE 11:- EQUITY
| a. | Composition of share capital: |
| December 31, 2018 | December 31, 2017 | |||||||||||||||
| Authorized | Issued and outstanding | Authorized | Issued and outstanding | |||||||||||||
| Number of Shares | ||||||||||||||||
| Ordinary shares of NIS 0.25 par value each | 80,000,000 | 40,399,290 | 80,000,000 | 33,295,618 | ||||||||||||
| b. | On December 3, 2015, a special general meeting of shareholders of the Company approved, in accordance with the majority required, a proposal to increase the Company’s authorized share capital by NIS 10,000,000 such that following the increase, the authorized share capital shall equal NIS 20,000,000 divided into 80,000,000 ordinary shares, par value NIS 0.25 each, and to amend the Company’s articles of association accordingly. |
| c. | Issued and outstanding capital: |
| Number of shares | NIS par value | |||||||
| Balance at December 31, 2016 | 28,156,728 | 7,039,182 | ||||||
| Issuance of share capital | 5,138,890 | 1,284,722 | ||||||
| Balance at December 31, 2017 | 33,295,618 | 8,323,904 | ||||||
| Issuance of share capital | 7,103,672 | 1,775,918 | ||||||
| Balance at December 31, 2018 | 40,399,290 | 10,099,822 | ||||||
F-35
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 11:- EQUITY (Cont.)
All ordinary shares have equal rights for all intent and purposes and each ordinary share confers its holder:
| 1. | The right to be invited and participate in all the Company’s general meetings, both annual and regular, and the right to one vote per ordinary share owned in all votes and in all Company’s general meeting participated. |
| 2. | The right to receive dividends if and when declared and the right to receive bonus shares if and when distributed. |
| 3. | The right to participate in the distribution of the Company’s assets upon liquidation. |
| d. | Issue of shares and warrants and changes in equity: |
| 1. | In September 2015, the Company completed a registered direct offering pursuant to which it sold an aggregate 2,068,966 ADSs representing 4,137,932 ordinary shares. In addition, the Company issued unregistered warrants to purchase 1,034,483 ADSs representing 2,068,966 ordinary shares. The offering (the “September 2015 Financing”) resulted in gross proceeds of USD 9,000. For further information regarding the warrants, please refer to Note 11.f.3. |
In October 2015, the Company completed a registered direct offering pursuant to which it sold an aggregate 1,109,196 ADSs representing 2,218,392 ordinary shares. In addition, the Company issued unregistered warrants to purchase 443,678 ADSs representing 887,356 ordinary shares. The offering (the “October 2015 Financing”) resulted in gross proceeds of USD 4,825. For further information regarding the warrants, please refer to Note 11.f.3.
As part of the September 2015 Financing, the Company also issued placement agent warrants to purchase 103,448 ADSs representing 206,897 ordinary shares exercisable at USD 5.25 per ADS (equivalent to USD 2.625 per ordinary share), subject to certain adjustments, for a period of five years. In addition, as part of the October 2015 Financing, the Company also issued placement agent warrants to purchase 55,460 ADSs representing 110,920 ordinary shares exercisable at USD 5.25 per ADS (equivalent to USD 2.625 per ordinary share), subject to certain adjustments, for a period of five years.
The investor warrants and placement agent warrants may be exercised on a cashless basis if six months after issuance there is no effective registration statement registering the ADSs underlying the warrants. The fair value of the placement agents warrants issued in the September 2015 Financing and October 2015 Financing at the grant date were USD 317 and USD 143, respectively and were considered as additional issuance costs.
F-36
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 11:- EQUITY (Cont.)
The cash issuance costs in relation to the September 2015 Financing and October 31, 2015 Financing were USD 789 and USD 525, respectively.
In relation to the September 2015 Financing and October 2015 Financing, the Company first allocated the proceeds to the warrants, and that due to the dollar exercise price terms and in accordance with IAS 39 the warrants are considered to be a freestanding liability instrument that is measured at fair value at each reporting date, based on its fair value, with changes in the fair values being recognized in the Company’s statement of comprehensive loss as financial income or expense. The remaining proceeds were allocated to the shares and were recorded to equity. The issuance costs were allocated between the warrants and the shares in proportion to the allocation of the proceeds.
The portions of the issuance costs that were allocated to the warrants and to the ordinary share were recorded as financial expense in the Company’s statement of comprehensive loss and to the additional paid in capital in the Company’s balance sheet, respectively.
| 2. | In January 2017, the Company completed a registered direct offering with certain institutional and accredited investors, pursuant to which it sold an aggregate 2,500,000 ADSs representing 5,000,000 of its ordinary shares and warrants to purchase 1,250,000 ADSs representing 2,500,000 of its ordinary shares for an aggregate purchase price of USD 5,000 (the “January 2017 Financing”). The warrants may be exercised after 6 months from the date of issuance for a period of five and a half years and have an exercise price of USD 2.25 per ADS (subject to certain adjustments). The Company also issued placement agent warrants to purchase 125,000 ADSs representing 250,000 ordinary shares exercisable at USD 2.25 per ADS, subject to certain adjustments, for a period of five years. The investor warrants and placement agent warrants may be exercised on a cashless basis if six months after issuance there is no effective registration statement registering the ADSs underlying the warrants. |
The issuance costs in relation to the January 2017 Financing was USD 621.
In relation to the January 2017 Financing, the Company first allocated the proceeds to the warrants, and that due to the dollar exercise price terms and in accordance with IAS 39 the warrants are considered to be a freestanding liability instrument that is measured at fair value at each reporting date, based on its fair value, with changes in the fair values being recognized in the Company’s statement of comprehensive loss as financial income or expense. The remaining proceeds were allocated to the shares and were recorded to equity. The issuance costs were allocated between the warrants and the shares in proportion to the allocation of the proceeds.
The portions of the issuance costs that were allocated to the warrants and to the ordinary share were recorded as financial expense in the Company’s statement of comprehensive loss and to the additional paid in capital in the Company’s balance sheet, respectively.
F-37
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 11:- EQUITY (Cont.)
The fair value of the warrants issued to the investors in the January 2017 Financing at the commitment date was USD 1,868. The fair value of the placement agents warrants issued in the January 2017 Financing at the grant date were USD 188, and were considered as additional issuance costs.
| 3. | In December 2017, the Company issued 69,445 ADSs representing 138,890 of its ordinary shares to one of its service providers for its services. |
| 4. | On March 13, 2018, the Company completed a registered direct offering with certain institutional investors, pursuant to which it sold an aggregate 3,333,336 ADSs representing 6,666,672 of its ordinary shares and warrants to purchase 2,500,002 ADSs representing 5,000,004 of its ordinary shares for an aggregate purchase price of USD 5,000. The warrants may be exercised after 6 months from the date of issuance for a period of five and a half years and have an exercise price of USD 2.00 per ADS (subject to certain adjustments). The Company also issued placement agent warrants to purchase 166,667 ADSs representing 333,334 ordinary shares exercisable at USD 2.00 per ADS, subject to certain adjustments, for a period of five years. |
| 5. | On March 9, 2018, 982,344 and 98,234 warrants as part of a March 2014 financing grant expired. |
| 6. | In May 2018, the Company issued 200,000 ADSs representing 400,000 ordinary shares to one of its service providers for its services. |
| 7. | In December 2018, the Company issued 18,500 ADSs representing 37,000 ordinary shares to one of its service providers for its services. |
| e. | Warrants classified as equity: |
| 1. | On March 31, 2014, 9,907,500 registered warrants (Series 7) that were exercisable into 396,300 ordinary shares of the Company expired. Accordingly, the Company recorded an amount of USD 258 as share premium. |
| 2. | As part of a March 2014 financing and December 2014 financing, the Company issued warrants. The warrants issued in the March 2014 Financing may be exercised after 6 months from the date of issuance for a period of four years and have an exercise price of USD 6.43 per ADS (equivalent to USD 3.215 per ordinary share) (subject to certain adjustments). The warrants issued in the December 2014 financing may be exercised for a period of five years following issuance and have an exercise price of USD 4.45 per ADS (equivalent to USD 2.225 per ordinary share) (subject to certain adjustments). The fair value of the warrants issued as part of the March 2014 financing as of commitment were USD 1,098. The fair value of the warrants issued as part of the December 2014 financing as of commitment were USD 1,535. |
| 3. | As mentioned in Note 11.d.1, the Company issued warrants as part of the September 2015 Financing and October 2015 Financing. These warrants may be exercised after 6 months from the date of issuance for a period of five and a half years and have an exercise price of USD 5.25 per ADS (equivalent to USD 2.625 per ordinary share) (subject to certain adjustments). |
F-38
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 11:- EQUITY (Cont.)
The fair value of the warrants issued as part of the September 2015 Financing as of commitment was USD 3,167. The fair value of the warrants issued as part of the October 2015 Financing as of commitment were USD 1,147.
| 4. | As mentioned in Note 11.d.2 the Company issued warrants as part of the January 2017 Financing. The fair value of the warrants issued as part of the January 2017 Financing as of commitment date was USD 1,868. |
| 5. | As mentioned in Note 11.d.4 the Company issued warrants as part of the March 2018 Financing. The fair value of the warrants issued as part of the January 2018 Financing as of commitment date was USD 3,593. |
| f. | Warrants classified as liability: |
The Company had 39,042,000 registered warrants (Series 10) that were exercisable into 1,561,680 ordinary shares of the Company for NIS 9.85 per share. The warrants were exercisable until October 31, 2017.
The fair value of the warrants (Series 10), as of December 31, 2015 and 2016 was USD 240 and USD 146, respectively. Changes in fair value of the warrants from commitment date to December 31, 2017 were recorded as financial income in the Company’s statement of comprehensive loss.
The Company had 37,372,500 registered warrants (Series 11) that were exercisable into 1,494,900 ordinary shares of the Company for NIS 9.80 per share. The warrants were exercisable until October 31, 2017.
The fair value of the warrants (Series 11), as of December 31, 2015 and 2016 were USD 307 and USD 126, respectively. Changes in fair value of the warrants from commitment date to December 31, 2017 were recorded as financial income in the Company’s statement of comprehensive loss.
The Company had 1,470,000 registered warrants (Series 12) that were exercisable into 1,470,000 ordinary shares of the Company for NIS 15.29 per share. The warrants were exercisable until October 31, 2017.
The fair value of the warrants (Series 12), as of December 31, 2015 and 2016 was USD 303 and USD 291, respectively. Changes in fair value of the warrants from commitment date to December 31, 2017 were recorded as financial income in the Company’s statement of comprehensive loss.
As described at Note 11.e.3, in September and October 2015 the Company issued warrants to purchase 2,275,863 and 998,276 of the Company’s ordinary shares, respectively.
On October 31, 2017 the registered warrants (Series 10,11,12) expired.
F-39
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 11:- EQUITY (Cont.)
| g. | Stock options: |
On November 28, 2013, the board of directors approved the adoption of the 2013 Share Option Plan (the “2013 Plan”). Under the 2013 Plan, the Company may grant its officers, directors, employees and consultants, stock options, of the Company. Each stock option granted shall be exercisable at such times and terms and conditions as the Board of Directors may specify in the applicable option agreement, provided that no option will be granted with a term in excess of 10 years.
Upon the adoption of the 2013 Plan the Company reserved for issuance 2,500,000 shares of ordinary shares, NIS 0.25 par value each. As of December 31, 2018, the Company had 1,215,000 shares available for future grant under the 2013 Plan.
NOTE 12:- SHARE-BASED PAYMENT TRANSACTIONS
| a. | Expenses recognized in the financial statements: |
Year ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| USD | ||||||||||||
| Research and development expenses | $ | 123 | $ | 139 | $ | 129 | ||||||
| General and administrative expenses | 130 | 53 | 180 | |||||||||
| $ | 253 | $ | 192 | $ | 309 | |||||||
| b. | Share-based payment transactions granted by the Company: |
| 1. | In February 2016, the Company’s board of directors approved a grant of unlisted options exercisable into 160,000 of the Company’s ordinary shares to three of its employees and one senior officer for an exercise price of NIS 4.317 per shares (USD 1.15 per share based on the exchange rate reported by the Bank of Israel on December 31, 2018). The options vest on quarterly basis for a period of 4 years from the grant date. |
| 2. | In May 2016, the Company’s board of directors approved a grant of 74,000 shares of the Company to a service provider. Pursuant to the agreement with the service provider, and as partial consideration, the Company issued 37,000 ordinary shares and agreed to issue an additional 37,000 ordinary shares within 180 days, provided that the agreement was not terminated. During 2016 the Company recorded an amount of USD 74 for share based payment expenses relating to this transaction. |
F-40
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 12:- SHARE-BASED PAYMENT TRANSACTIONS (Cont.)
| 3. | On May 26, 2016 the Company’s board of directors approved a grant of 20,000 options exercisable into 20,000 ordinary shares of the Company to one of its advisers at an exercise price of 5.376 NIS per share (USD 1.43 per share based on December 31, 2018 exchange rate). The options will vest on a quarterly basis for a period of 4 years from the grant date. |
| 4. | In March 2017, the Company’s board of directors approved a grant of unlisted options exercisable into 210,000 of the Company’s ordinary shares to three of its employees for an exercise price of NIS 3.662 per share (USD 0.97 per share based on December 31, 2018 exchange rate). The options vest on a quarterly basis for a period of 48 months from the grant date. |
| 5. | In May 2017, the Company’s board of directors approved a grant of unlisted options exercisable into 60,000 of the Company’s ordinary shares to an advisor for an exercise price of NIS 3.393 per share (USD 0.90 per share based on the exchange rate reported by the Bank of Israel on December 31, 2018). The options vest on a quarterly basis for a period of 48 months from the grant date. |
| 6. | In December 2017, the Company’s board of directors approved a grant of unlisted options exercisable into 460,000 and 240,000 of the Company’s ordinary shares to the Company’s employees and directors, respectively, for an exercise price of NIS 2.513 and NIS 2.926 per share, respectively (USD 0.67 and USD 0.78 per share, respectively, based on the exchange rate reported by the Bank of Israel on December 31, 2018). The options vest on a quarterly basis for a period of 48 months from the grant date. |
| c. | Movement during the year: |
The following table lists the number of share options, their weighted average exercise prices and modification in option plans of employees, directors and consultants for the periods indicated:
| Shares subject to options outstanding | ||||||||||||||||||||||||
| 2018 | 2017 | 2016 | ||||||||||||||||||||||
| Number | Weighted average exercise price | Number | Weighted average exercise price | Number | Weighted average exercise price | |||||||||||||||||||
| USD | USD | USD | ||||||||||||||||||||||
| Outstanding at beginning of year | 1,490,423 | 1.35 | 737,028 | 2.63 | 798,579 | 3.14 | ||||||||||||||||||
| Grants | - | - | 970,000 | 0.75 | 180,000 | 1.15 | ||||||||||||||||||
| Forfeited/expired | (53,023 | ) | 7.53 | (216,605 | ) | 4.26 | (241,551 | ) | 3.32 | |||||||||||||||
| Outstanding at end of year | 1,437,400 | 1.20 | 1,490,423 | 1.35 | 737,028 | 2.63 | ||||||||||||||||||
| Exercisable at end of year | 736,155 | 2.41 | 451,266 | 2.41 | 402,500 | 3.87 | ||||||||||||||||||
| d. | The weighted average remaining contractual life for the shares subject to options outstanding as of December 31, 2018, 2017 and 2016 was 7.64 years, 8.38 years and 8.86 years, respectively. |
F-41
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 12:- SHARE-BASED PAYMENT TRANSACTIONS (Cont.)
| e. | The range of exercise prices for shares subject to options outstanding as of December 31, 2018, 2017 and 2016 was between USD 0.07 and USD 8.32. |
| f. | The fair value of the Company’s share options granted for the years ended December 31, 2016 and 2017 was estimated using the binomial option pricing model using the following assumptions: |
| December 31, | ||||||||
| Description | 2017 | 2016 | ||||||
| Risk-free interest rate | 1.89%-2.31% | 2.01%-2.02% | ||||||
| Expected volatility | 74.4%-77.64% | 77.84%-78.22% | ||||||
| Dividend yield | 0 | 0 | ||||||
| Contractual life | 10 | 10 | ||||||
| Early Exercise Multiple (Suboptimal Factor) | 2.5 | 2.5 | ||||||
| Exercise price | 0.72-0.98 | 1.12-1.39 | ||||||
NOTE 13:- RESEARCH AND DEVELOPMENT EXPENSES
Year ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| USD | ||||||||||||
| Clinical and preclinical trials | $ | 4,768 | $ | 3,809 | $ | 5,104 | ||||||
| Salary and related expenses | 909 | 888 | 664 | |||||||||
| Patents | 229 | 234 | 198 | |||||||||
| Royalties | 14 | 11 | 11 | |||||||||
| Laboratory materials | 73 | 63 | 38 | |||||||||
| Rent | 51 | 51 | 48 | |||||||||
| Depreciation | 3 | 3 | 4 | |||||||||
| Others | 28 | 47 | 48 | |||||||||
| $ | 6,075 | $ | 5,106 | $ | 6,115 | |||||||
NOTE 14:- GENERAL AND ADMINISTRATIVE EXPENSES
Year ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| USD | ||||||||||||
| Professional services | $ | 1,199 | $ | 1,132 | $ | 1,022 | ||||||
| Investors and public relations | 400 | 338 | 511 | |||||||||
| Salary and related expenses | 665 | 611 | 413 | |||||||||
| Directors’ fee | 173 | 198 | 212 | |||||||||
| Rent | 34 | 34 | 32 | |||||||||
| Travel | 170 | 112 | 196 | |||||||||
| Insurance | 282 | 214 | 142 | |||||||||
| Stock exchange fees | 58 | 62 | 57 | |||||||||
| Office and computer maintenance | 82 | 81 | 68 | |||||||||
| Vehicle maintenance | 16 | 14 | 14 | |||||||||
| Depreciation | 11 | 16 | 14 | |||||||||
| Others | 69 | 56 | 52 | |||||||||
| $ | 3,159 | $ | 2,868 | $ | 2,733 | |||||||
F-42
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 15:- FINANCE INCOME
Year ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| USD | ||||||||||||
| Finance expenses: | ||||||||||||
| Bank commissions | $ | 18 | $ | 28 | $ | 27 | ||||||
| Financial expenses from defined benefit plans | - | 12 | ||||||||||
| Interest expenses from IFRS 15 implementation | 427 | - | - | |||||||||
| Net loss from exchange rate fluctuations | 115 | 588 | 16 | |||||||||
| Other loss from long-term investment revaluation | 644 | 5 | - | |||||||||
| 1,204 | 621 | 55 | ||||||||||
| Finance income: | ||||||||||||
| Interest income on bank deposits | (51 | ) | (69 | ) | (89 | ) | ||||||
| Net change in fair value warrants exercisable into shares | - | (564 | ) | (285 | ) | |||||||
| (51 | ) | (633 | ) | (374 | ) | |||||||
| $ | 1,153 | $ | (12 | ) | $ | (319 | ) | |||||
NOTE 16:- LOSS PER SHARE
| a. | Details of the number of shares and loss used in the computation of loss per share: |
Year ended December 31, | ||||||||||||||||||||||||
| 2018 | 2017 | 2016 | ||||||||||||||||||||||
| Weighted number of shares | Loss | Weighted number of shares | Loss | Weighted number of shares | Loss | |||||||||||||||||||
| USD | USD | USD | ||||||||||||||||||||||
| Number of shares and loss used in the computation of basic and diluted loss per share | 38,902,214 | 6,571 | 32,525,138 | 6,339 | 27,692,668 | 8,257 | ||||||||||||||||||
| b. | To compute diluted loss per share for the year ended December 31, 2018, the total number of 1,437,400 shares subject to outstanding unlisted options have not been taken into account since they have anti-dilutive effect. |
F-43
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 17:- TAXES ON INCOME
| a. | Corporate tax rates: |
Israeli taxation:
Corporate tax rate in Israel in 2018 was 23%, 2017 - 24% and in 2016 was 25%.
On January 4, 2016, the Israeli Parliament’s Plenum approved by a second and third reading the Bill for Amending the Income Tax Ordinance (No. 217) (Reduction of Corporate Tax Rate), 2015, which consists of the reduction of the corporate tax rate from 26.5% to 25%.
In December 2016, the Israeli Parliament approved the Economic Efficiency Law (Legislative Amendments for Applying the Economic Policy for the 2017 and 2018 Budget Years), 2016 which reduces the corporate income tax rate to 24% (instead of 25%) effective from January 1, 2017 and to 23% effective from January 1, 2018
The Company estimates that the effect of the change in tax rates will have no impact on the financial statements.
| b. | Final tax assessments: |
The Company received final tax assessments through 2013.
The related company, Eye-Fite, tax assessment through 2013 is considered as final.
| c. | Net operating carryforward losses for tax purposes and other temporary differences: |
As of December 31, 2018 the Company and Eye-Fite had carryforward losses amounting to approximately USD 99,715 and Nil, respectively.
| d. | Deferred taxes: |
The Company did not recognize deferred tax assets for carryforward losses and other temporary differences because their utilization in the foreseeable future is not probable.
NOTE 18:- TRANSACTIONS WITH RELATED PARTIES
| a. | The related parties of the Company are associates, subsidiaries, directors and key management personnel of the Group, and a close member of the family of any of the persons mentioned above. |
| b. | The Chairman of the Company’s board of directors is a senior partner in the patent firm which represents the Company in intellectual property and commercial matters (the “Service Provider”). The Service Provider charges the Company for services it renders on an hourly basis. |
F-44
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 18:- TRANSACTIONS WITH RELATED PARTIES (Cont.)
| c. | Composition of balances with related parties for the year ended December 31, 2018, and each of the three years then ended: |
Year ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| USD | ||||||||||||
| Management and consulting fees (including bonuses) (1) | $ | 471 | $ | 505 | $ | 322 | ||||||
| Other expenses and share-based payment (1) | $ | 50 | $ | 79 | $ | 158 | ||||||
| Patent expenses | $ | 229 | $ | 234 | $ | 198 | ||||||
| Directors’ fee and share-based payment (2) | $ | 173 | $ | 171 | $ | 134 | ||||||
| (1) Number of related parties | $ | 1 | $ | 1 | $ | 1 | ||||||
| (2) Number of directors | $ | 5 | $ | 5 | $ | 5 | ||||||
| d. | Eye-Fite License agreement and Services Agreement: |
A license agreement was entered into between the Company and Eye-Fite (the “Eye-Fite License Agreement”) according to which the Company granted Eye-Fite a non-transferrable exclusive license for the use of the Company’s know-how solely in the field of ophthalmic diseases for research, development, commercialization and marketing throughout the world.
In addition to the Eye-Fite License Agreement, the Company, OpthhaliX and Eye-Fite entered into a services agreement (the “Services Agreement”) pursuant to which the Company provided management services with respect to all pre-clinical and clinical research studies, production and supply of the compounds related to the Eye-Fite License Agreement and payment for consultants that are listed in the agreement for their involvement in the clinical trials and in all the activities leading up to, and including, the commercialization of CF101 for ophthalmic indications.. The Company granted Eye-Fite an exclusive license to use these inventions in the field of ophthalmic diseases around the world at no consideration. The Eye-Fite License and Service Agreement were terminated during 2017 in connection with the Merger, please refer to note 1.b.
F-45
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In thousands (except for share and per share data)
NOTE 19:- SUBSEQUENT EVENTS
| a. | On January 18, 2019, the Company completed a registered direct offering with an institutional investor, pursuant to which it sold an aggregate 2,238,096 ADSs representing 4,476,192 of its ordinary shares and warrants to purchase 2,238,096 ADSs representing 4,476,192 of its ordinary shares for an aggregate purchase price of USD 2,350. The warrants have an exercise price of $1.30 per ADS, are immediately exercisable and expire five and one-half years from the issuance date. |
| b. | On February 25, 2019, the Company’s Distribution Agreement with CKD was amended to expand the exclusive right to distribute Namodenoson for the treatment of NASH in addition to liver cancer in South Korea. CKD has agreed to pay the Company up to an additional USD 3,000 in upfront and milestone payments payable with respect to the NASH indication. The Company will also be entitled to a transfer price for delivering finished product to CKD. | |
| c. | On March 11, 2019, a Special General Meeting of shareholders of the Company approved a grant of unlisted options exercisable into 400,000 of the Company’s ordinary shares to Company’s chief executive officer for an exercise price of NIS 2.344 per share (USD 0.67 per share based on the exchange rate reported by the Bank of Israel on December 31, 2018). The options vest on a quarterly basis for a period of 48 months from the date of approval by the Company’s Board of Directors on January 7, 2019. |
F-46