Exhibit 99.2

Can - Fite Presentation – January 2016
This presentation contains forward - looking statements, about Can - Fite’s expectations, beliefs or intentions regarding, among other things, its product development efforts, business, financial condition, results of operations, strategies or prospects . In addition, from time to time, Can - Fite or its representatives have made or may make forward - looking statements, orally or in writing . Forward - looking statements can be identified by the use of forward - looking words such as “believe,” “expect,” “intend,” “plan,” “may,” “should” or “anticipate” or their negatives or other variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical or current matters . These forward - looking statements may be included in, but are not limited to, various filings made by Can - Fite with the U . S . Securities and Exchange Commission (the “SEC”), press releases or oral statements made by or with the approval of one of Can - Fite’s authorized executive officers . Forward - looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made . Because forward - looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause Can - Fite’s actual results to differ materially from any future results expressed or implied by the forward - looking statements . Many factors could cause Can - Fite’s actual activities or results to differ materially from the activities and results anticipated in such forward - looking statements, including, but not limited to, the factors summarized in Can - Fite’s filings with the SEC and in its periodic filings with the Tel - Aviv Stock Exchange . Forward Looking Statement 2
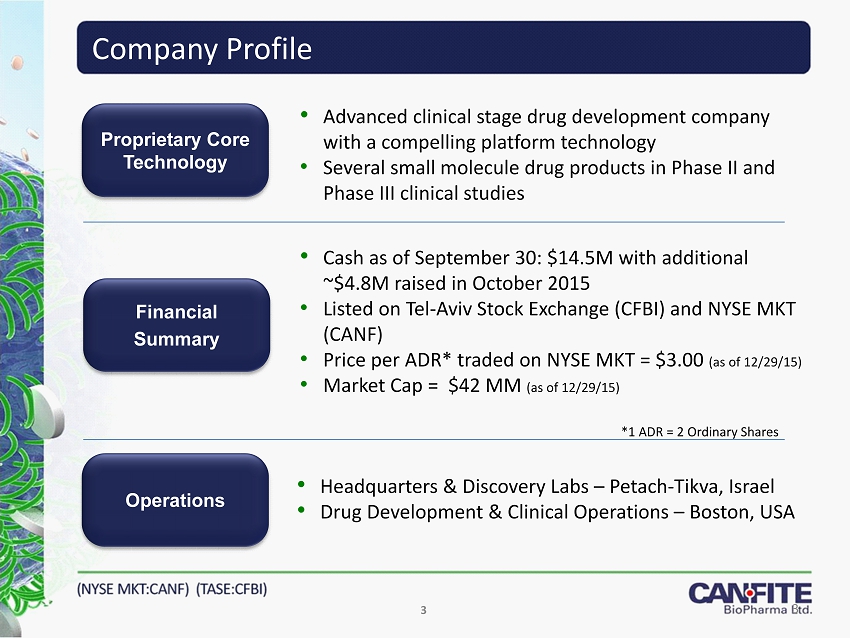
3 Company Profile Operations Financial Summary Proprietary Core Technology • Headquarters & Discovery Labs – Petach - Tikva , Israel • Drug Development & Clinical Operations – Boston, USA • Advanced clinical stage drug development company with a compelling platform technology • Several small molecule drug products in Phase II and Phase III clinical studies • Cash as of September 30: $ 14.5M with additional ~$ 4.8M raised in October 2015 • Listed on Tel - Aviv Stock Exchange (CFBI) and NYSE MKT (CANF) • Price per ADR* traded on NYSE MKT = $3.00 (as of 12/29/15) • Market Cap = $ 42 MM (as of 12/29/15) *1 ADR = 2 Ordinary Shares 3
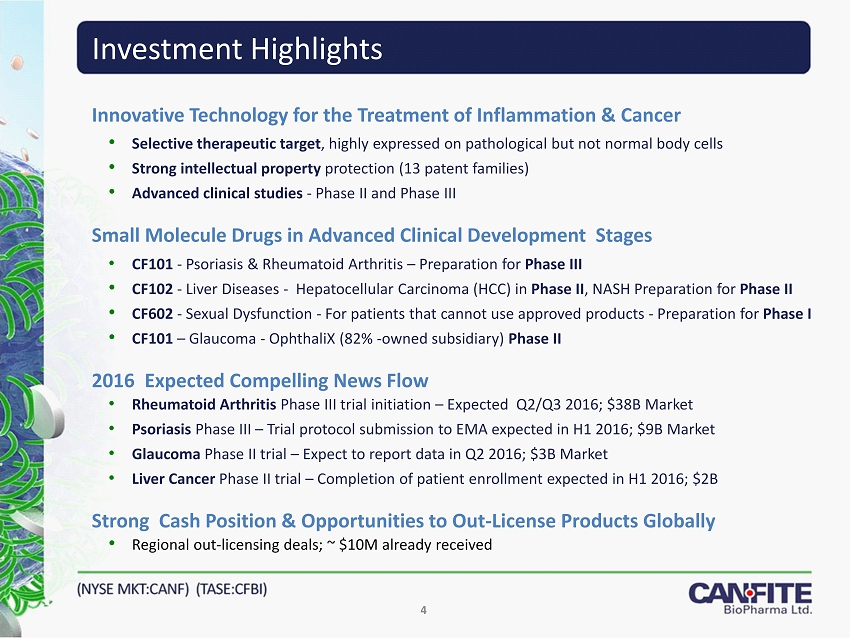
Investment Highlights Innovative Technology for the Treatment of Inflammation & Cancer • Selective therapeutic target , highly expressed on pathological but not normal body cells • Strong intellectual property protection (13 patent families) • Advanced clinical studies - Phase II and Phase III Small Molecule Drugs in Advanced Clinical Development Stages • CF101 - Psoriasis & Rheumatoid Arthritis – Preparation for Phase III • CF102 - Liver Diseases - Hepatocellular Carcinoma (HCC) in Phase II , NASH Preparation for Phase II • CF602 - Sexual Dysfunction - For patients that cannot use approved products - Preparation for Phase I • CF101 – Glaucoma - OphthaliX (82% - owned subsidiary) Phase II 2016 Expected Compelling News Flow • Rheumatoid Arthritis Phase III trial initiation – Expected Q2/Q3 2016; $38B Market • Psoriasis Phase III – Trial protocol submission to EMA expected in H1 2016; $9B Market • Glaucoma Phase II trial – Expect to report data in Q2 2016; $3B Market • Liver Cancer Phase II trial – Completion of patient enrollment expected in H1 2016; $2B Strong Cash Position & Opportunities to Out - License Products Globally • Regional out - licensing deals; ~ $10M already received 4

Corporate Partnerships Regional out - licensing deals - ~$ 10 million received to date [Traded on South Korean Stock Exchange (Ticker : A 009290 )] • Exclusive regional license to develop and commercialize CF101 for the treatment of rheumatoid arthritis in Korea • Up to $1.5 M in upfront and milestone payments ($0.5M received to date) • 7% royalties 5 [Traded on Nasdaq (Ticker : CPHR ) ; TSX : ( Ticker : CPH] • Exclusive regional license to distribute CF101 for the treatment of rheumatoid arthritis and moderate to severe psoriasis in Canada • Up to CDN$3.65M in upfront and milestone payments ( CDN$1.65M received to date) • 16.5% royalties
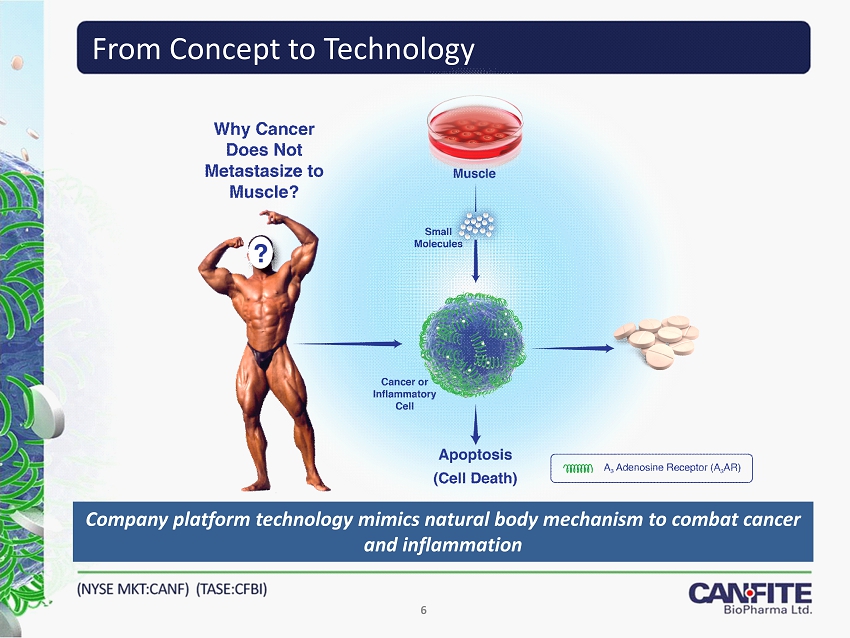
From Concept to Technology 6 Company platform technology mimics natural body mechanism to combat cancer and inflammation
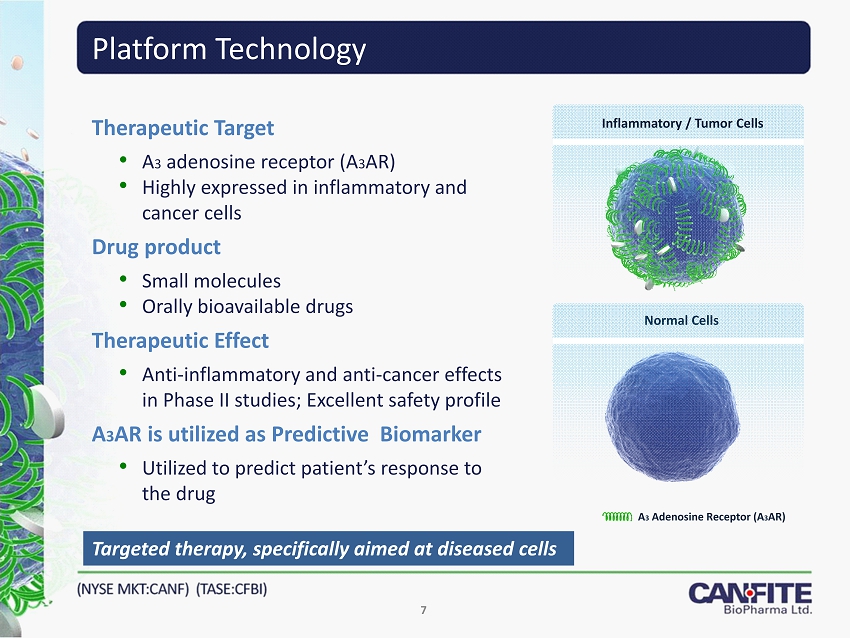
Therapeutic Target • A 3 adenosine receptor (A 3 AR ) • Highly expressed in inflammatory and cancer cells Drug product • Small molecules • Orally bioavailable drugs Therapeutic Effect • Anti - inflammatory and anti - cancer effects in Phase II studies; Excellent safety profile A 3 AR is utilized as Predictive Biomarker • Utilized to predict patient’s response to the drug Platform Technology Targeted therapy, specifically aimed at diseased cells Inflammatory / Tumor Cells Normal Cells A 3 Adenosine Receptor (A 3 AR) 7
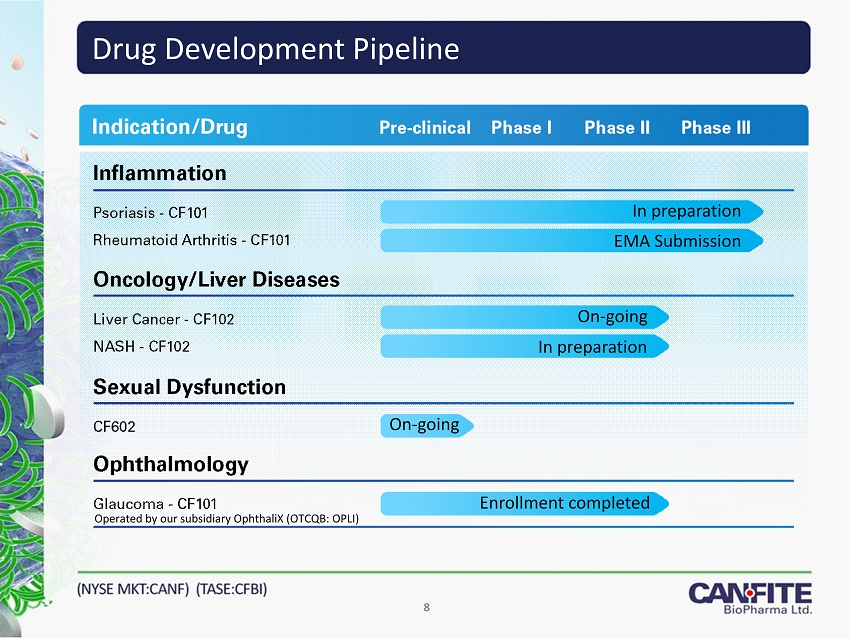
Drug Development Pipeline 8 Operated by our subsidiary OphthaliX (OTCQB: OPLI) In preparation EMA Submission On - going Enrollment completed On - going In preparation
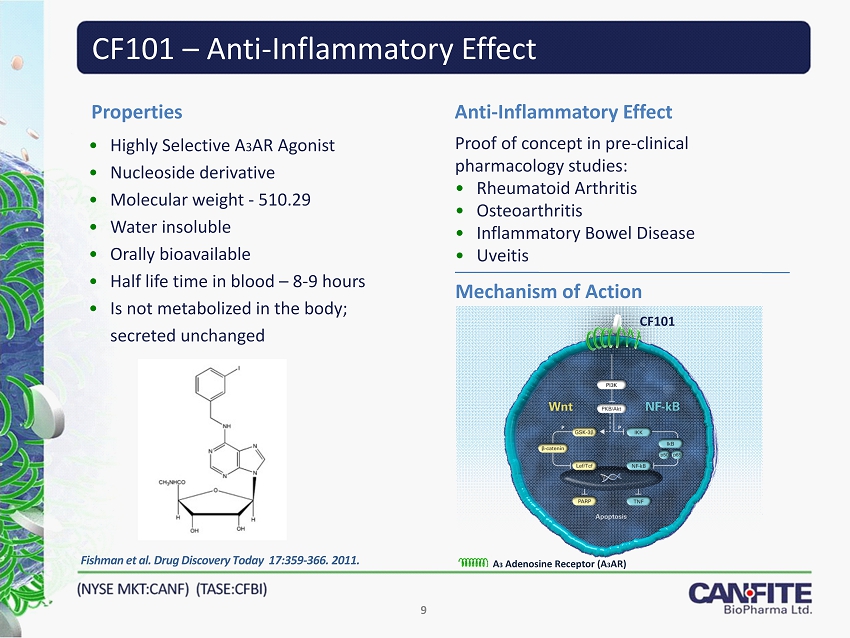
CF101 – Anti - Inflammatory Effect • Highly Selective A 3 AR Agonist • Nucleoside derivative • Molecular weight - 510.29 • Water insoluble • Orally bioavailable • Half life time in blood – 8 - 9 hours • Is not metabolized in the body; secreted unchanged Properties Proof of concept in pre - clinical pharmacology studies: • Rheumatoid Arthritis • Osteoarthritis • Inflammatory Bowel Disease • Uveitis Anti - Inflammatory Effect Mechanism of Action Fishman et al . Drug Discovery Today 17:359 - 366. 2011. CF101 A 3 Adenosine Receptor (A 3 AR) 9
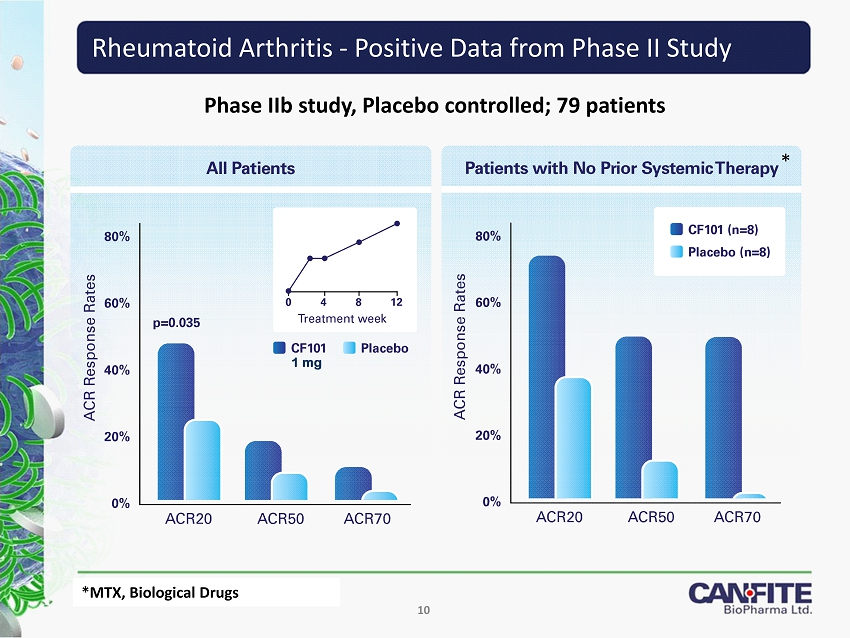
Phase IIb study, Placebo controlled; 79 patients Rheumatoid Arthritis - Positive Data from Phase II Study 10 * *MTX, Biological Drugs *MTX, Biological Drugs 1 mg
Rheumatoid Arthritis – Phase III Study 11 *MTX, Biological Drugs *MTX, Biological Drugs Phase III - Study Protocol • A Phase III trial to evaluate the efficacy and safety of CF101 when a dded to conventional t herapy in the treatment of rheumatoid a rthritis • 3 arms: 1 mg & 2 mg of CF101 and placebo • Number of patients planned : 456; 152 per study arm • Study duration: 16 weeks • Primary End Point: ACR20 at 16 weeks of treatment Regulatory Status • Successful meeting with the Medical Products Agency (MPA) in Sweden • IRB submission of Phase III protocol in Israel • EMA submission expected in Q1 2016
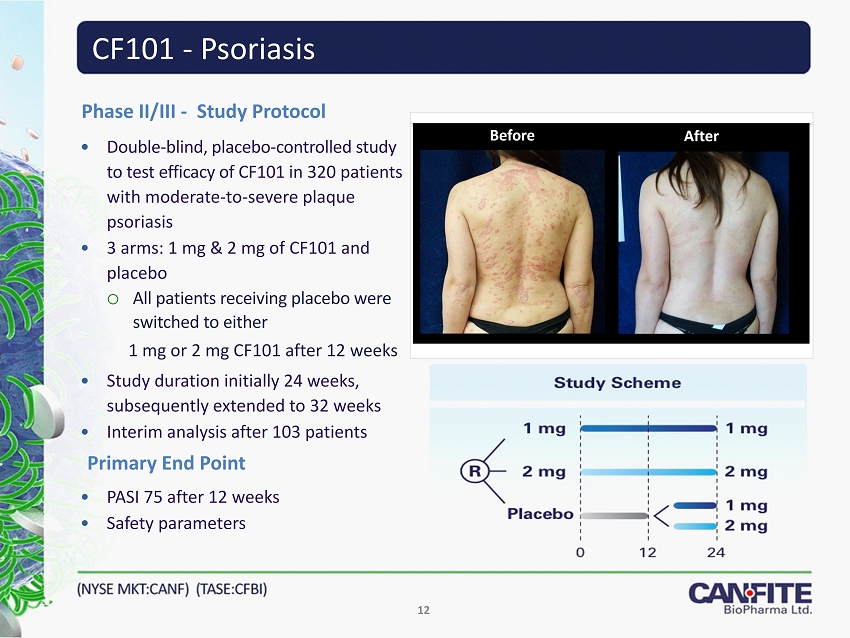
CF101 - Psoriasis • Double - blind, placebo - controlled study to test efficacy of CF101 in 320 patients with moderate - to - severe plaque psoriasis • 3 arms: 1 mg & 2 mg of CF101 and placebo o All patients receiving placebo were switched to either 1 mg or 2 mg CF101 after 12 weeks • Study duration initially 24 weeks, subsequently extended to 32 weeks • Interim analysis after 103 patients Phase II/III - Study Protocol • PASI 75 after 12 weeks • Safety parameters Primary End Point 12 Before After
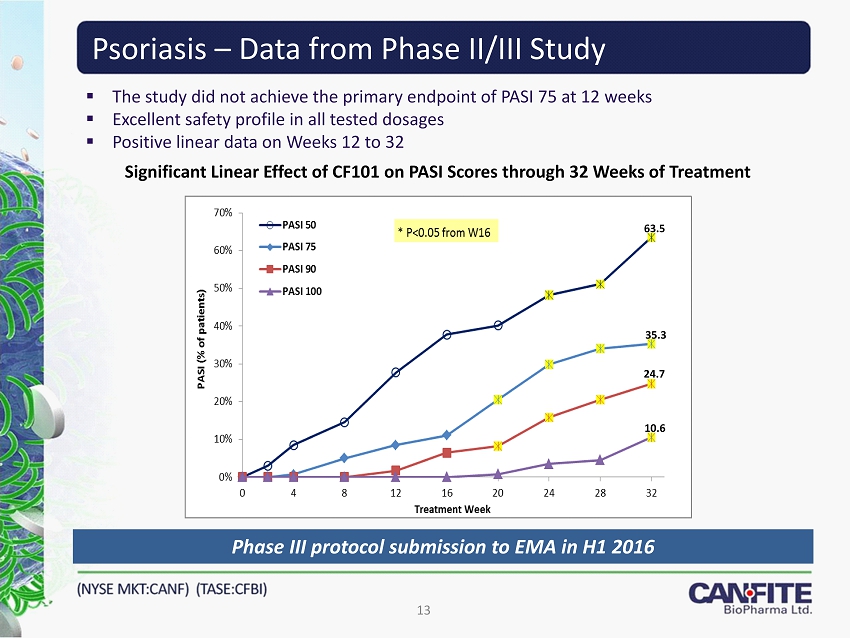
Psoriasis – Data from Phase II/III Study ▪ The study did not achieve the primary endpoint of PASI 75 at 12 weeks ▪ Excellent safety profile in all tested dosages ▪ Positive linear data on Weeks 12 to 32 Significant Linear Effect of CF101 on PASI Scores through 32 Weeks of Treatment 13 63.5 35.3 24.7 10.6 Phase III protocol submission to EMA in H 1 2016
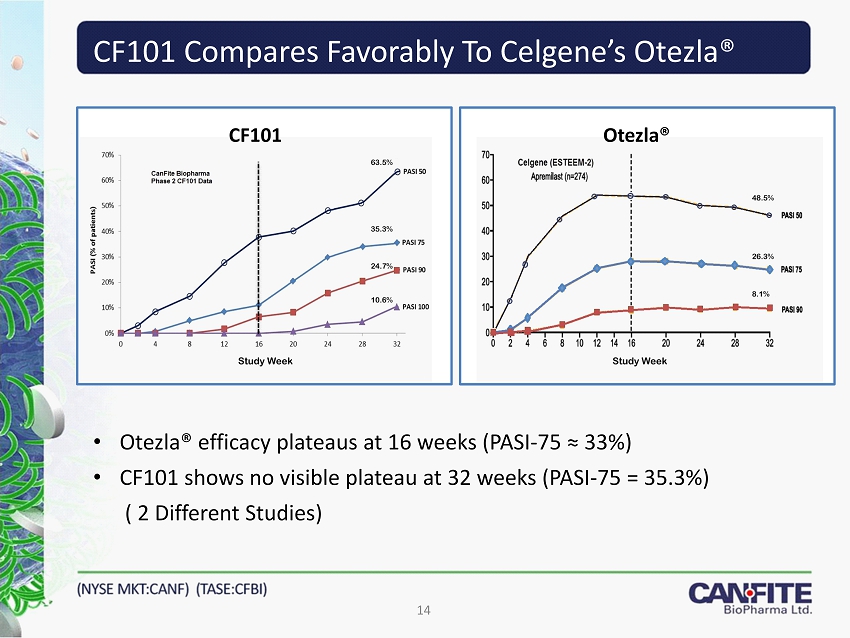
CF101 Compares Favorably To Celgene’s Otezla® 14 • Otezla® efficacy plateaus at 16 weeks (PASI - 75 ≈ 33 %) • CF 101 shows no visible plateau at 32 weeks (PASI - 75 = 35.3 %) ( 2 Different Studies) Otezla® CF101
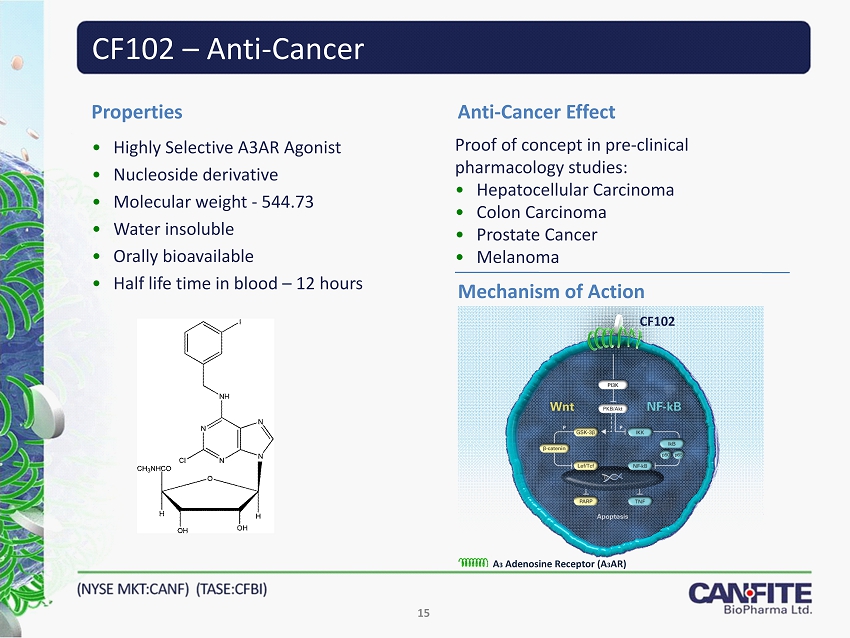
CF102 – Anti - Cancer • Highly Selective A3AR Agonist • Nucleoside derivative • Molecular weight - 544.73 • Water insoluble • Orally bioavailable • Half life time in blood – 12 hours Properties Proof of concept in pre - clinical pharmacology studies: • Hepatocellular Carcinoma • Colon Carcinoma • Prostate Cancer • Melanoma Anti - Cancer Effect Mechanism of Action CF102 A 3 Adenosine Receptor (A 3 AR) 15
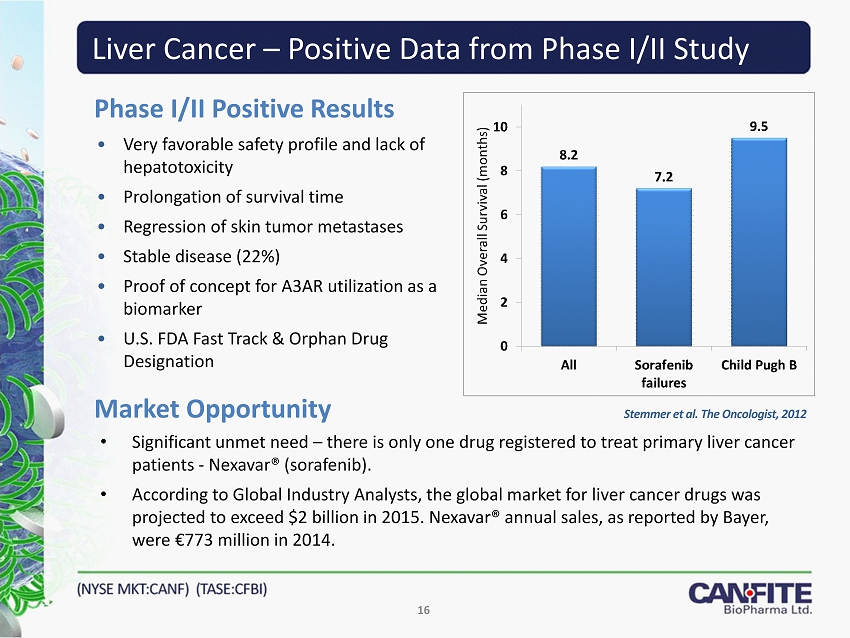
Liver Cancer – Positive Data from Phase I/II Study • Very favorable safety profile and lack of hepatotoxicity • Prolongation of survival time • Regression of skin tumor metastases • Stable disease (22%) • Proof of concept for A3AR utilization as a biomarker • U.S. FDA Fast Track & Orphan Drug Designation Market Opportunity 16 Phase I/II Positive Results • Significant unmet need – there is only one drug registered to treat primary liver cancer patients - Nexavar ® (sorafenib). • According to Global Industry Analysts, the global market for liver cancer drugs was projected to exceed $2 billion in 2015. Nexavar® annual sales, as reported by Bayer, were € 773 million in 2014. Stemmer et al. The Oncologist, 2012

Phase II - Study Protocol • Second - Line Treatment • Advanced Hepatocellular Carcinoma; Child - Pugh B • 78 patients; • US, Europe and Israel • Primary end point: overall survival Study Status • Regulatory approvals to commence Phase II study were received in the US, Europe and Israel • Patient enrollment for a global Phase II study has been initiated • Completion of patient e nrollment in Phase II s tudy expected in H1 2016 Liver Cancer – Phase II Global Study Ongoing 17 Orphan Drug Designation US & EU Fast Track Designation
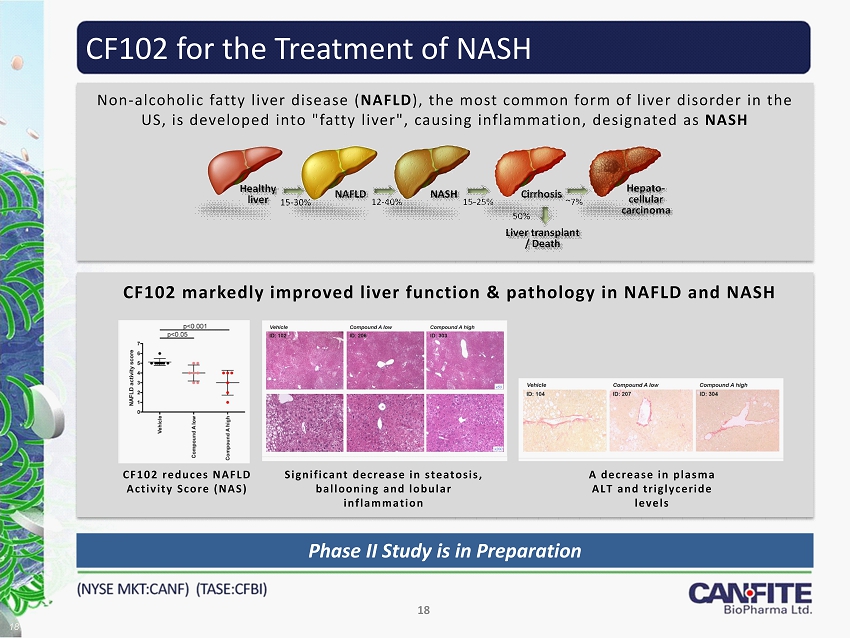
CF102 for the Treatment of NASH 18 CF102 markedly improved liver function & pathology in NAFLD and NASH CF102 reduces NAFLD Activity Score (NAS ) Significant decrease in steatosis, ballooning and lobular inflammation A decrease in plasma ALT and triglyceride levels Non - alcoholic fatty liver disease ( NAFLD ), the most common form of liver disorder in the US, is developed into "fatty liver", causing inflammation, designated as NASH Phase II Study is in Preparation
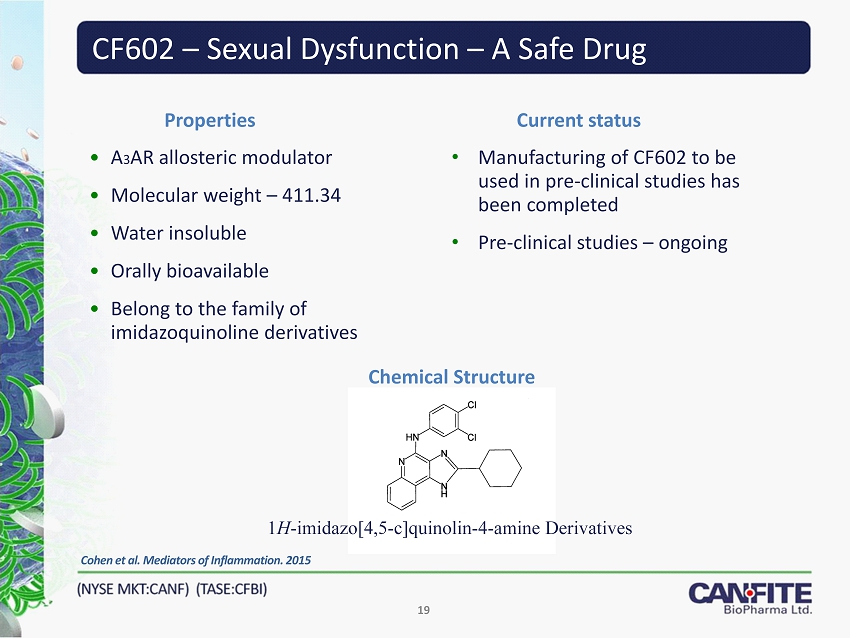
CF 602 – Sexual Dysfunction – A Safe Drug 19 Properties Cohen et al . Mediators of Inflammation. 2015 • A 3 AR allosteric modulator • Molecular weight – 411.34 • Water insoluble • Orally bioavailable • Belong to the family of imidazoquinoline derivatives Current status • Manufacturing of CF 602 to be used in pre - clinical studies has been completed • Pre - clinical studies – ongoing Chemical Structure 1 H - imidazo[ 4 , 5 - c]quinolin - 4 - amine Derivatives
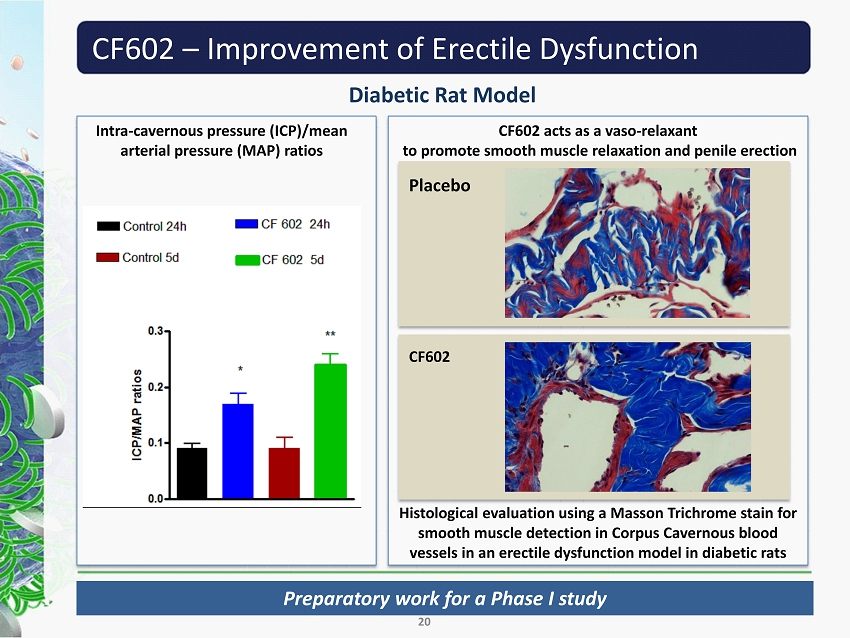
CF602 – Improvement of Erectile Dysfunction Diabetic Rat Model 20 Intra - cavernous pressure (ICP)/ mean arterial pressure (MAP) ratios CF 602 acts as a vaso - relaxant to promote smooth muscle relaxation and penile erection Placebo CF602 Histological evaluation using a Masson Trichrome stain for smooth muscle detection in Corpus Cavernous blood vessels in an erectile dysfunction model in diabetic rats Preparatory work for a Phase I study
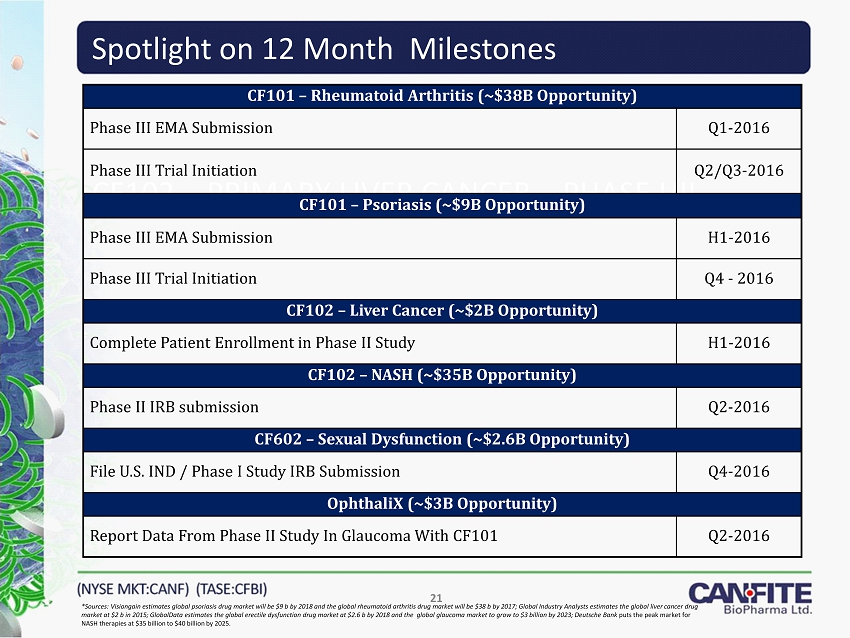
Spotlight on 12 Month Milestones CF102
– PRIMARY LIVER CANCER – PHASE I/II 21 *Sources: Visiongain estimates global psoriasis drug market will be $ 9 b by 2018 and the global rheumatoid arthritis drug market will be $ 38 b by 2017 ; Global Industry Analysts estimates the global liver cancer drug market at $ 2 b in 2015 ; GlobalData estimates the global erectile dysfunction drug market at $ 2.6 b by 2018 and the global glaucoma market to grow to $ 3 billion by 2023 ; Deutsche Bank puts the peak market for NASH therapies at $ 35 billion to $ 40 billion by 2025 . CF101 – Rheumatoid Arthritis (~$38B Opportunity) Phase III EMA Submission Q1 - 2016 Phase III Trial I nitiation Q2/Q3 - 2016 CF101 – Psoriasis (~$9B Opportunity) Phase III EMA Submission H1 - 2016 Phase III Trial Initiation Q4 - 2016 CF102 – Liver Cancer (~$2B Opportunity) Complete Patient Enrollment in Phase II Study H1 - 2016 CF102 – NASH (~$35B Opportunity) Phase II IRB submission Q2 - 2016 CF602 – Sexual Dysfunction (~$2.6B Opportunity) File U.S. IND / Phase I Study IRB Submission Q4 - 2016 OphthaliX (~$3B Opportunity) Report Data From Phase II Study In Glaucoma With CF101 Q2 - 2016