Exhibit 4.34
CERTAIN PORTIONS OF THIS EXHIBIT HAVE BEEN OMITTED AND ARE SUBJECT TO A CONFIDENTIAL TREATMENT REQUEST. COPIES OF THIS EXHIBIT CONTAINING THE OMITTED INFORMATION HAVE BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION. THE OMITTED PORTIONS OF THIS DOCUMENT ARE MARKED WITH A […].
CAN-FITE BIOPHARMA LTD
AND
CIPHER PHARMACEUTICALS INC.
DISTRIBUTION AND SUPPLY AGREEMENT
DATED AS OF MARCH 20, 2015
TABLE OF CONTENTS
| Page | |||
| 1. | DEFINITIONS | 1 | |
| 2. | DISTRIBUTION RIGHTS | 11 | |
| 2.1 | Exclusive Distributorship and License | 11 | |
| 2.2 | Restrictions on Marketing of Products | 12 | |
| 2.3 | Covenant Not to Market Competing Products | 12 | |
| 2.4 | Authorized Generics | ||
| 3. | MARKETING | 12 | |
| 3.1 | Marketing Decisions | 12 | |
| 3.2 | Marketing Plan | 12 | |
| 3.3 | Advertising and Promotion | 13 | |
| 3.4 | Information Sharing | 13 | |
| 3.5 | Reports | 13 | |
| 4. | REGULATORY MATTERS AND PRODUCT DEVELOPMENT | 14 | |
| 4.1 | Registration Responsibilities | 14 | |
| 4.2 | Development Responsibilities | 14 | |
| 4.3 | Post-Approval Regulatory Responsibilities | 15 | |
| 4.4 | Other Approvals | 15 | |
| 4.5 | Monitoring ADE and Quality Complaint | 15 | |
| 4.6 | Quality and Technical Agreement | 15 | |
| 4.7 | Pharmacovigilance Agreement | 16 | |
| 4.8 | Cooperation | 16 | |
| 4.9 | Joint Steering Committee | 16 | |
| 5. | ADES, PRODUCT QUALITY AND PRODUCT RECALLS | 16 | |
| 5.1 | ADEs | 16 | |
| 5.2 | Product Complaints other than ADEs | 17 | |
| 5.3 | Product Recall | 17 | |
| 5.4 | Cooperation as to ADE, Product Inquiries and Recalls | 18 | |
| 6. | PURCHASE PRICE AND SUPPLY OF PRODUCTS | 19 | |
| 6.1 | Supply of Products | 19 | |
| 6.2 | Forecasts, Orders | 20 | |
| 6.3 | Continuity of Supply | 22 | |
| 6.4 | Method of Delivery of Supplied Product | 23 | |
| 6.5 | Acceptance, Rejection and Revocation of Acceptance. | 23 | |
| 6.6 | Rejection Procedures | 23 | |
| 6.7 | Prices and Payments | 24 | |
| 6.8 | Audit | 25 | |
| - ii - |
| 6.9 | Facility Audits | 26 | |
| 7. | INTELLECTUAL PROPERTY | 26 | |
| 7.1 | Ownership of Can-Fite Intellectual Property | 26 | |
| 7.2 | Ownership of Distributor Intellectual Property | 26 | |
| 7.3 | Maintenance and Prosecution of Product Patents | 27 | |
| 7.4 | Notice of Patent Infringement | 27 | |
| 7.5 | Can-Fite Trademarks Indemnified Infringement Claims | 28 | |
| 7.6 | Trademarks Indemnified Infringement Claims | 28 | |
| 7.7 | Infringement of Product Technology by a Third Party | 29 | |
| 7.8 | Trademarks | 30 | |
| 8. | CONFIDENTIALITY | 31 | |
| 8.1 | Can-Fite’s Information | 31 | |
| 8.2 | Distributor’s Information | 31 | |
| 8.3 | Exceptions | 32 | |
| 8.4 | Publications | 32 | |
| 8.5 | Survival | 33 | |
| 9. | TERM AND TERMINATION OF AGREEMENT | 33 | |
| 9.1 | Term | 33 | |
| 9.2 | Termination | 33 | |
| 9.3 | Accrued Rights, Surviving Obligations | 35 | |
| 9.4 | Transitional Matters | 35 | |
| 9.5 | Transfer of Approvals | 35 | |
| 9.6 | Effect of Termination | 36 | |
| 9.7 | License Survival During Bankruptcy | 36 | |
| 10. | INDEMNITY | 37 | |
| 10.1 | Indemnification by Can-Fite | 37 | |
| 10.2 | Indemnification by Distributor | 37 | |
| 10.3 | Procedure | 38 | |
| 10.4 | Indemnification Not Sole Remedy | 38 | |
| 10.5 | Insurance | 39 | |
| 11. | REPRESENTATIONS, WARRANTIES AND COVENANTS; LIMITATIONS OF LIABILITY | 39 | |
| 11.1 | Representations, Warranties and Covenants | 39 | |
| 11.2 | Quality Assurance Representations, Warranties and Covenants | 40 | |
| 11.3 | Distributor’s Compliance with Laws | 41 | |
| 11.4 | Limitation of Liability | 42 | |
| 12. | MISCELLANEOUS | 42 | |
| 12.1 | Governing Law | 42 | |
| - iii - |
| 12.2 | Dispute Resolution | 42 | |
| 12.3 | Entire Agreement; Amendments | 43 | |
| 12.4 | Tax Withholding | 43 | |
| 12.5 | Notices | 43 | |
| 12.6 | Assignment | 44 | |
| 12.7 | Public Announcements | 45 | |
| 12.8 | Severance | 45 | |
| 12.9 | Non-Waiver | 45 | |
| 12.10 | Further Assurances | 45 | |
| 12.11 | Force Majeure | 45 | |
| 12.12 | Anti-Corruption | 46 | |
| 12.13 | Disclaimer of Agency | 46 | |
| 12.14 | Construction | 46 | |
| 12.15 | Counterparts | 47 | |
| 12.16 | Consents in Writing | 47 |
| Schedule A - CAN-FITE TRADEMARKS AND PATENTS |
| Schedule B - SPECIFICATIONS |
| Schedule C - PAYMENTS TO CAN-FITE |
| Schedule D - MINIMUM SALES REQUIREMENTS |
DISTRIBUTION AND SUPPLY AGREEMENT
between
CAN-FITE BIOPHARMA LTD
and
CIPHER PHARMACEUTICALS INC.
This Distribution and Supply Agreement (the “Agreement”) is entered into as of March 20, 2015, (the “Effective Date”) by and between Can-Fite BioPharma Ltd. (“Can-Fite”), an Israeli company located at 10 Bareket Street, Kiryat Matalon, PO Box 7537, Petah-Tikva, 49170, Israel, and Cipher Pharmaceuticals Inc. (“Distributor”), an Ontario corporation located at 5650 Tomken Road Unit 16, Mississauga Ontario L4W 4P1. Unless otherwise specified, all capitalized terms shall have the meaning specified in Article 1 herein.
RECITALS
| 1. | Can-Fite has the exclusive rights to certain know-how and intellectual property rights relating to the Product; |
| 2. | Can-Fite is still in Clinical Development of the Product for the treatment of psoriasis and rheumatoid arthritis and has yet to receive the requisite Approval for the marketing and sale of any therapeutic products which include the Product; |
| 3. | Can-Fite wishes, once the Clinical Development is successfully completed and the Approvals are obtained, to have the Product manufactured and packaged for distribution, marketing and sale for use in the Field in the Territory; |
| 4. | Distributor has experience in the distribution, marketing and sale of pharmaceutical products in the Territory; and |
| 5. | Can-Fite desires to grant Distributor and Distributor desires to accept, the right and obligation to distribute and sell Product for use in the Field in the Territory subject to the terms and conditions of this Agreement. |
NOW THEREFORE THIS AGREEMENT WITNESSES THAT in consideration of the foregoing and the covenants and promises contained in this Agreement, the Parties agree as follows:
1. DEFINITIONS
As used herein, the following terms shall have the following meanings:
(a) “AB Rated Generic” means a Third Party’s product which is deemed by Health Canada to be the therapeutic equivalent of the Product and which contains the same Active Ingredient as the Product.
| - 2 - |
(b) “Act” means the Canada Food and Drugs Act, as amended from time to time.
(c) “Active Ingredient” means a pharmaceutical compound which is intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment or prevention of disease or to affect the structure or function of the body.
(d) “Additional Quantity” has the meaning set forth in Section 6.2(b)(iii).
(e) “Adverse Drug Experience” or “ADE” means any untoward medical occurrence in a patient administered Product and which does not necessarily have a causal relationship with the Product. An ADE can therefore be any unfavorable and unintended sign (for example, an abnormal laboratory finding), symptom, or disease temporally associated with the use of the Product, whether or not considered related to the Product (ICH E2D).
(f) “Affiliate” means, with respect to any Party, any other Person directly or indirectly controlling or controlled by, or under direct or indirect common control with, such Party. For purposes of this definition, a Person shall be deemed to “control” any other Person if it owns or controls a sufficient interest in the voting equity (or other comparable ownership if the other Person is not a corporation) such that it can direct, order or control the actions of such other Person and the ownership of fifty percent (50%) or more of the voting equity (or other comparable ownership if the other Person is not a corporation) shall be conclusive evidence of control.
(g) “Alternate Supplier” means any Third Party alternate supplier of the Product that Can-Fite used to supply Product for jurisdictions outside the Territory.
(h) “Approvals” means collectively the Regulatory Approval and the Other Approvals.
(i) “Approved Manufacturer” means Can-Fite and/or a Third Party approved in advance in writing by Distributor (which approval will not be unreasonably withheld or delayed), for the purpose of operating an Approved Manufacturing Site to Manufacture the Supplied Product; provided that all references to Approved Manufacturer(s) shall be intended to include any Alternate Suppliers.
(j) “Approved Manufacturing Site” means a manufacturing site at which Supplied Product may be Manufactured, Packaged or Tested in full compliance with the applicable Approvals and all applicable Laws and in the case of an Approved Manufacturer, other than Distributor, or their respective Affiliates, approved in advance in writing by Can-Fite, which approval will not be unreasonably delayed or withheld.
(k) “Approved Transaction” has the meaning set forth in Section 8.3.
(l) “Authorities” means collectively the Regulatory Authority and the Other Authorities.
| - 3 - |
(m) “Authorized Generic” means the Product marketed as a generic product by Distributor as contemplated by Section 2.4 hereof.
(n) “Business Day” means any day other than a Saturday, a Sunday, or a day on which banks in the Province of Ontario, Canada or in Tel-Aviv, Israel are required or authorized to close.
(o) “Can-Fite Indemnitees” means any of Can-Fite and Can-Fite’s Approved Manufacturers and their respective Affiliates, subsidiaries, equity holders, directors, managers, officers, employees, trustees, representatives, consultants, sublicensees, agents, successors and permitted assigns.
(p) “Can-Fite Trademarks” means any trademark, trade name, trade dress, logo, design or associated artwork owned by or licensed to Can-Fite pertaining to the Product, including that listed in Schedule A.
(q) “Claims” has the meaning set forth in Section 7.5.
(r) “Clinical Development” means clinical studies conducted by Can-Fite or its Affiliates or Sublicensees to seek Regulatory Approval for the Product.
(s) “COA” has the meaning set forth in Section 6.5(a).
(t) “Commercially Reasonable Efforts” means exercising such reasonable efforts and diligence in accordance with a Party’s reasonable business, legal, medical and scientific judgment and in accordance with the efforts and resources a pharmaceutical company similar to the relevant Party would use for a pharmaceutical product which is of similar market potential at a similar stage of its product life, taking into account the competitiveness of the marketplace, the proprietary position of the product and the potential profitability of the product.
(u) “Competing Product” means any branded formulations which contain N6-(3-iodobenzyl)-adenosine- 5’-N-methyluronamide (“IB-MECA”) as an Active Ingredient or any AB Rated Generic.
(v) “Confidential Information” means all Intellectual Property and confidential facts relating to the business and affairs of a Party or any of its Affiliates, including financial information, business opportunities, information relating to pharmaceutical products of any nature whatsoever (including Product Information in the case of Can-Fite), know-how (including Product Know-How in the case of Can-Fite), and compilations of information in any form whatsoever; provided, however, that “Confidential Information” shall not include any information that (a) was already in the public domain at the time of disclosure, (b) becomes part of the public domain through no action or omission of the receiving Party after disclosure to the receiving party, (c) was already known to the receiving Party, other than under an obligation of confidentiality to the disclosing party, at the time of the disclosure by the other Party, as shown by independent written proof, (d) was independently discovered or developed by the receiving Party without the use of Confidential Information belonging to the disclosing Party as shown by pre-existing written proof, or (e) was disclosed to the receiving Party, other than under an obligation of confidentiality to which a Third Party was subject, by a Third Party who had no obligation to the disclosing Party not to disclose such information to others, as shown by independent written proof.
| - 4 - |
(w) “Contract Finisher” means a Person engaged by any of Can-Fite, an Approved Manufacturer or Distributor to be responsible for Packaging and/or Testing Supplied Product in the Territory.
(x) “Deadline Date” has the meaning set forth in Section 6.2(b)(ii).
(y) “Dispute” has the meaning set forth in Section 12.2.
(z) “Distributor Indemnitees” means any of Distributor and Distributor’s SubDistributors and each of their respective Affiliates, subsidiaries, equity holders, directors, managers, officers, employees, trustees, representatives, consultants, sublicensees, agents, successors and permitted assigns.
(aa) “Enforcement Action” has the meaning set forth in Section 7.7(a).
(bb) “FDA” means the United States Food and Drug Administration or any successor agency which issues a Regulatory Approval for the Marketing of the Product in the United States.
(cc) “Field” means the oral, intravenous and topical use of the Product for treating psoriasis and rheumatoid arthritis in humans and animals.
(dd) “Firm Order” has the meaning set forth in Section 6.2(a)(i).
(ee) “First Commercial Sale” means the date of the first arm’s length sale of a Supplied Product by Distributor, its Affiliates or SubDistributors to a Third Party in the Territory, as evidenced by delivery of the Supplied Product to the Third Party.
(ff) “Fiscal Year” means the twelve (12) months ending December 31.
(gg) “Force Majeure” means an event or circumstances beyond the reasonable control of a Party or Approved Manufacturer or Contract Finisher and which, with the exercise of diligent efforts that Party or their Approved Manufacturer or Contract Finisher is unable to prevent, including Acts of God, government restrictions, wars, insurrections, failure of suppliers, subcontractors and carriers, strikes, labor disputes, failures of electricity supply and inability to obtain essential ingredients or supplies (for the avoidance of doubt, the Parties agree that the failure of any Approved Manufacturer or a Contract Finisher to supply Can-Fite shall not be deemed a Force Majeure with respect to Can-Fite except to the extent such failure to supply is the direct result of a Force Majeure applicable to such Approved Manufacturer or such Contract Finisher).
(hh) “Forecast” has the meaning set forth in Section 6.2(a)(i).
| - 5 - |
(ii) “Good Manufacturing Practices (GMP)” means at any time the quality systems and good manufacturing practices as required by applicable Laws, directives, rules, regulations, guides and guidance in existence in the Territory at that time.
(jj) “Health Canada” means Health Canada or any successor agency which issues a Regulatory Approval for the Marketing of the Product in the Territory.
(kk) “IFRS” means International Financial Reporting Standards.
(ll) “Indemnified Party”
has the meaning set forth in Section 10.3.
(mm) “Indemnifying Party” has the meaning set forth in Section 10.3.
(nn) “Initial Milestone Payment” has the meaning set for in Part A of Schedule C.
(oo) “Initial Term” has the meaning set forth in Section 9.1.
(pp) “Intellectual Property” means all patents (including the Product Patents in the case of Can-Fite’s Intellectual Property), copyrights, trademarks, service marks, service names, trade names, internet domain names, e-mail addresses, applications or registrations for any of the foregoing, or extensions, renewals, continuations or re-issues thereof, or amendments or modifications thereto, brandmarks, brand names, trade dress, labels, logos, know-how (including the Product Know-How in the case of Can-Fite’s Intellectual Property), show-how, technical and non-technical information, trade secrets, formulae, techniques, sketches, drawings, models, inventions, designs, specifications, processes, apparatus, equipment, databases, research, experimental work, development, pharmacology and clinical data, software programs and applications, software source documents, Third-Party licenses, and any similar type of proprietary intellectual property right vesting in the owner and/or licensee thereof pursuant to the applicable Laws of any relevant jurisdiction or under any applicable license or contract, whether now existing or hereafter created, together with all modifications, enhancements and improvements thereto.
(qq) “Joint Steering Committee” has the meaning set forth in Section 4.9.
(rr) “Law” means all laws, statutes, ordinances, decrees, judgments, codes, standards, acts, orders, by-laws, rules, regulations, permits, legally binding policies and guidelines and legally binding requirements of all Regulatory and Other Authorities including the Canada Food and Drugs Act, including any amendments thereto, and all regulations, rules, guidelines and procedures promulgated thereunder, as well as analogous legislation in the remainder of the Territory.
(ss) “Latent Defect” means a defect that existed at the time that title to Supplied Product passed to Distributor which could not have been detected by Distributor utilizing the Distributor’s usual and customary inspection procedures for incoming finished product intended for distribution in the Territory, which in any event will be in accordance with Distributor’s GMP obligations.
| - 6 - |
(tt) “License Agreement” means the Patent License Agreement between Can-Fite and the National Institutes of Health designated as L-249-2001/0 together with the First Amendment thereto designated as L-249-2001/1 and the Second Amendment thereto designated as L-249-2001/2.
(uu) “Losses” has the meaning set forth in Section 10.1.
(vv) “Manufacture” means to make Supplied Product in compliance with GMP, including to process, prepare, make and Test the raw materials used in the preparation of Supplied Product and to Test the Supplied Product prior to release for Packaging, in each case in a finished dosage form ready for administration to humans and “Manufacturing” has a corresponding meaning.
(ww) “Market” means to promote, advertise, distribute, market, offer to sell and/or sell for purposes of a commercial sale, and “Marketing” has a corresponding meaning.
(xx) “Marketing Plan” has the meaning set forth in Section 3.2.
(yy) “Milestone Payments” means the Milestone Payments, as set forth in Schedule C.
(zz) “NDS” or “New Drug Submission” means any regulatory submission made by Distributor for Regulatory Approval to Market the Supplied Product, as the same may be amended or supplemented and any related or successor NDS) and shall include all accompanying data and information including supplements and amendments.
(aaa) “Net Profits” means with respect to any Authorized Generic authorized to be commercialized by Distributor hereunder [...].
(bbb) “Net Sales” means, for any period, the aggregate gross amounts invoiced by Distributor, its Affiliates or SubDistributors in connection with sales of the Product to arm’s length Third Parties, (excluding sales of the Distributor to its SubDistributors or Affiliates) for use in the Field in the Territory, less any and all (a) customary discounts or incentives of any type or nature, (such as, without limitation, trade, quantity and cash discounts, charge-backs, recalls, actual returns or rebates or other similar adjustments relating to credits issued to arm’s length Third Parties (excluding sales of the Distributor to its SubDistributors or Affiliates)) on such sales, which specifically relate to the Product and are recognized in accordance with Canadian generally accepted accounting principles, (b) customary freight shipping, insurance costs, duties and taxes paid by Distributor or its Affiliates or a SubDistributor on shipment of Product to Distributor, and (c) to the extent not included above, payments under Section 6.7(b). No deductions shall be made for commissions paid to individuals, whether they be with independent sales agencies or regularly employed by Distributor, its Affiliates or SubDistributors, and on its payroll, or for the cost of collections. Notwithstanding the foregoing, in the event Distributor launches an Authorized Generic as contemplated pursuant to Section 2.4 herein, Net Sales for such Authorized Generic shall, in addition to the deductions provided above, allow for discounts and rebates customary in the generic industry that are consistent with Distributor’s ordinary course of business in the Territory, provided such discounts and rebates relate solely to the sale of the Authorized Generic.
| - 7 - |
(ccc) “Net Selling Price” means the average selling price per Unit of Product by Distributor, its Affiliates and SubDistributors for use in the Field in the Territory to arm’s length Third Party customers (excluding sales of the Distributor to its Affiliates and SubDistributors) of Supplied Product calculated on a monthly basis by dividing the Net Sales of Supplied Product in a calendar month by the number of Units sold in that calendar month in the Territory.
(ddd) “Official Body” means any national, federal, provincial or local government or government of any subdivision thereof, or any parliament, legislature, council, agency, authority, board, commission, department, bureau or instrumentality thereof, or any court, tribunal, grand jury, mediator or arbitrator, whether foreign or domestic, in each case having jurisdiction in the relevant circumstances.
(eee) “Other Approvals” means, for the Product, the approval or authorization granted by the Other Authorities for the Marketing of the Product for use in the Field in the Territory, including the Pricing Approval and the Reimbursement Approval, as applicable.
(fff) “Other Authorities” means Official Bodies (other than the Regulatory Authority) whose approval is required by applicable Law to Market and/or obtain reimbursement for Supplied Product in a jurisdiction in the Territory.
(ggg) “Package” means to package and label Supplied Product for Marketing and “Packaging” has a corresponding meaning.
(hhh) “Parties” means Can-Fite and Distributor, and “Party” means either of Can-Fite or Distributor, as the context requires.
(iii) “Person” means any individual, partnership, limited partnership, joint venture, syndicate, sole proprietorship, company or corporation with or without share capital, unincorporated association, trust, trustee, executor, administrator or other legal personal representative or other entity or Official Body.
(jjj) “Prevailing Party” has the meaning set forth in Section 12.2.
(kkk) “Pricing Approval” means any approval or authorization of any Official Body establishing prices for the Product in a jurisdiction for use in the Field in the Territory.
(lll) “Product” means the pharmaceutical preparations described in the Product Patents or associated with the Product Know-How, namely as N6 -(3-iodobenzyl)-adenosine- 5’-N-methyluronamide (IB-MECA) in an oral formulation delivered in bulk or final finished Packaged form for use in humans and meeting the Specifications, as the same may be amended or supplemented, and any related or successor NDSs filed with respect to the same initial application. “Product” also includes any future formulations containing IB-MECA and improvements thereof approved by Health Canada.
| - 8 - |
(mmm) “Product Information” means all in-vivo or clinical, non-clinical, pharmacology, toxicology, safety and efficacy data, stability data, formulary submissions, pharmaco-economic data, and other such information now or hereafter known and available to Distributor or Can-Fite or their respective Affiliates, SubDistributors or Approved Manufacturer(s) or their Affiliates, whether generally known to others or not.
(nnn) “Product Know-How” means the data, information, expertise, trade secrets, manufacturing, mixing and production procedures, technical assistance, and shop rights, known to, in the possession of or licensed to Can-Fite, its Affiliates or any Approved Manufacturer(s) or its Affiliates, whether generally known to others or not, and relating to the Manufacturing, Packaging, Marketing and/or Testing of Supplied Product, including:
(i) characteristics, selection of properties and data relating to materials, such as excipients, used or useful in the Manufacturing, Packaging and/or Testing of Supplied Product;
(ii) techniques, equipment and methods used or useful in the Manufacturing, Packaging or Testing of Supplied Product;
(iii) equipment and data relating to the Manufacturing, Packaging or Testing of Supplied Product; and
(iv) all in vivo or clinical, pharmacology, toxicology, safety and efficacy data, formulary submissions, pharmaco-economic data, and other such information useful or required in preparing applications for or obtaining or maintaining Regulatory Approval and/or for the Manufacturing, Packaging, Marketing and/or Testing of Supplied Product.
(ooo) “Product Liability Claim” means any Third Party claim involving any actual or alleged death or bodily or emotional injury arising out of or relating to any Supplied Product sold in the Territory for use in the Field.
(ppp) “Product Patents” means all patents owned by Can-Fite (i) which have issued as of the Effective Date or (ii) which issue at any time from applications pending as of the Effective Date, or from applications subsequently filed during the Term of this Agreement, which (in the case of both (i) and (ii)) claim, disclose or pertain to inventions necessary or useful for the Manufacture, use, import or Marketing of the Product, including any continuation, division, continuation-in-part, and any provisional applications, and which patents have not expired or been held invalid or unenforceable by a court of competent jurisdiction in a final, non-appealable decision, including all substitutions, extensions, registrations, confirmations, re-examinations, reissues or renewals of such patents. Schedule A lists, as of the Effective Date, all such Patents that have issued and pending applications and Schedule A shall be amended from time to time to include patents or applications owned by or licensed to Can-Fite or one or more of its Affiliates to the extent they claim inventions necessary or useful for Manufacturing, use, import, or Marketing of the Product within the Territory or an amendment to any Product Patents.
(qqq) “Product Technology” means collectively Product Know-How and Product Patents.
| - 9 - |
(rrr) “Regulatory Approval” means all approvals or authorizations granted by a Regulatory Authority for the Marketing of Supplied Product for use in the Field in the Territory.
(sss) “Regulatory Authority” means Health Canada, and/or any equivalent, similar or successor Official Body, whose approval is required by applicable Law to Market, Manufacture, Test and/or Package Supplied Product in any jurisdiction which forms part of the Territory.
(ttt) “Regulatory Requirements” means all applicable Regulatory Approvals, licenses, registrations, GMPs, and authorizations and all other requirements of any applicable Regulatory Authorities in relation to Supplied Product, including each of the foregoing which is necessary for, or otherwise governs, the Manufacture, Marketing, Packaging and Testing of Supplied Product for use in the Field in the Territory.
(uuu) “Reimbursement Approval” means any approval or authorization of any Official Body establishing a health insurance or drug reimbursement scheme for Supplied Product in a jurisdiction for use in the Field in the Territory.
(vvv) “Rejection Notice” has the meaning set forth in Section 6.5(b).
(www) “Renewal Term” has the meaning set forth in Section 9.1.
(xxx) “Responsible Person” has the meaning set forth in Section 12.2.
(yyy) “Resumption Notice” has the meaning set forth in Section 6.3(b).
(zzz) “Royalty Percentage” means 16.5% of Net Sales.
(aaaa) “Rules” has the meaning set forth in Section 12.2.
(bbbb) “Schedules” means the following Schedules to this Agreement (as the same may be amended from time to time in accordance with this Agreement):
| Schedule A | - | Can-Fite Trademarks and Patents | |
| Schedule B | - | Specifications | |
| Schedule C | - | Payments to Can-Fite | |
| Schedule D | - | Minimum Sales Requirements |
(cccc) “Serious ADEs” has the meaning set forth in Section 5.1.
(dddd) “Specifications” means the specifications of Supplied Product as set forth in Schedule B.
(eeee) “Stipulated Rejection Period” has the meaning set forth in Section 6.5(b).
(ffff) “SubDistributors” means Third Parties appointed by Distributor pursuant to Section 2.1(c) to Market Supplied Product for use in the Field in the Territory, but shall not include wholesalers, retailers, hospitals, government purchasers or managed and/or care organizations.
| - 10 - |
(gggg) “Supplied Product” means the Product and/or the Authorized Generic, whether or not it is supplied by Can-Fite; provided, however, that any representations, warranties or covenants of Can-Fite herein which refers to Supplied Product only relates to Supplied Product supplied by or on behalf of Can-Fite.
(hhhh) “Supply Interruption” has the meaning set forth in Section 6.3(a).
(iiii) “Tax(es)” means, with respect to Distributor, all federal, provincial, local, county, foreign and other taxes or government charges constituting sales, use, transfer, value added, customs, duty or excise taxes payable by the Distributor in connection with the importation or sale of Supplied Product.
(jjjj) “Term” has the meaning set forth in Section 9.1.
(kkkk) “Territory” means Canada.
(llll) “Test” means to test a product or its ingredients prior to release for further processing or for shipping and Marketing in compliance with applicable Law and “Testing” has the corresponding meaning.
(mmmm) “Third Party” means any Person other than Can-Fite, Distributor or their respective Affiliates.
(nnnn) “Third Party Laboratory” means the Third Party Laboratory selected jointly by the Parties for the purpose of adjudicating between them on the matters in disagreement under Sections 5.3(e), 6.6(c) and 6.6(d) below.
(oooo) “Trademarks” means any trademark, trade name, trade dress, logo, design or associated artwork selected, owned and/or used by Distributor or its Affiliates pertaining to Supplied Product, other than the Can-Fite Trademarks.
(pppp) “Transfer Price” means (i) Can-Fite’s cost (as determined in accordance with IFRS) of Manufacturing the Supplied Product, in the event Can-Fite is Manufacturing Supplied Product, or (ii) Can-Fite’s actual out-of-pocket cost and expense to procure the Supplied Product from an Approved Manufacturer or Contract Finisher as established from time to time, in the event Can-Fite is procuring Product from an Approved Manufacturer or Contract Finisher, except that if the Approved Manufacturer is an Alternate Supplier, the transfer price shall be no higher than that in effect prior to a Supply Disruption.
(qqqq) “Unit” means a single capsule or tablet of Supplied Product.
| - 11 - |
2. DISTRIBUTION RIGHTS
2.1 Exclusive Distributorship and License.
(a) Upon and subject to the terms and conditions of this Agreement, Can-Fite hereby appoints Distributor as its exclusive distributor of the Product for use in the Field in the Territory throughout the Term with the right and obligation to Market the Product for use in the Field in the Territory and the right to register and develop the Product pursuant to Sections 4.1 and 4.2. Can-Fite represents and warrants to Distributor that, except for the exclusive license granted in this Section 2.1, Can-Fite has not granted any other licenses to use, Market and/or import, the Products for use in the Field in the Territory. The term “exclusive” as used in this Section 2.1(a) means the rights granted to Distributor in this Section 2.1 are to the exclusion of all other persons and entities including Can-Fite. Can-Fite shall take all reasonable and prudent actions to ensure that the Product does not enter the Territory for use in the Field as black market goods.
(b) Distributor shall obtain exclusively from Can-Fite all Supplied Product for Marketing for use in the Field in the Territory, except as otherwise permitted by the terms of this Agreement. Can-Fite shall supply the Supplied Product to Distributor for Marketing by Distributor for use in the Field in the Territory in accordance with the terms of this Agreement and shall not supply the Supplied Product to any other Person for use in the Field in the Territory or to any Person, unless such Person agrees not to knowingly directly or indirectly through Third Parties sell the Supplied Product for use in the Field in the Territory.
(c) Distributor shall have the right to appoint SubDistributors to Market the Supplied Product for use in the Field solely within the Territory, and Distributor shall cause such SubDistributors to perform the applicable obligations of Distributor under this Agreement, or otherwise ensure that such obligations are performed by the Distributor. Distributor shall remain fully responsible and liable to Can-Fite for the performance of all of the terms of this Agreement by its SubDistributors. Distributor shall not be entitled to appoint as a SubDistributor any Person which is or has engaged in, directly or indirectly, developing or Marketing any Competing Product in the Territory.
(d) Can-Fite hereby grants to Distributor an exclusive license (including the right to grant sublicenses to SubDistributors) for use in the Field in the Territory to (i) all Product Technology necessary or useful in order to Market the Supplied Product, (ii) clinical, non-clinical and safety data in order to obtain Regulatory Approval, and Pricing Approval or Other Approvals in the Territory, and (iii) a Canadian URL for the Product.
(e) Except as expressly provided in this Agreement, Can-Fite is not granting to Distributor any right, title or interest, whether express or implied, in the Product or any Intellectual Property or other right that Can-Fite or its Affiliates may own or control.
(f) Notwithstanding anything contained herein, Can-Fite is not granting to Distributor any rights under the License Agreement.
2.2 Restrictions on Marketing of Products.
From and after the Effective Date, Distributor shall not, and shall cause its Affiliates and SubDistributors to not, Market or export the Supplied Product outside the Territory or outside the Field, or Market or export the Supplied Product to any Person who, to the knowledge of any of Distributor, its SubDistributors, or its Affiliates, intends to directly or indirectly Market or export Product outside the Territory or outside the Field.
| - 12 - |
2.3 Covenant Not to Market Competing Products.
From and after the Effective Date until the earlier of termination of this Agreement or the launch of an AB Rated Generic by a Third Party within the Territory, Distributor shall not, and shall cause its Affiliates and SubDistributors to not Market a Competing Product in the Territory.
Can-Fite agrees that during the Term neither it nor its Affiliates will Market and/or import, the Product or any Competing Product for use in the Field in the Territory, nor license or otherwise authorize any Third Party to develop, Market, Manufacture for sale inside the Territory for use in the Field, and/or import the Product or any Competing Product for use in the Field in the Territory.
2.4 Authorized Generics.
In the event that a Third Party launches an AB Rated Generic in the Territory, Distributor may sell an Authorized Generic for use in the Field in the Territory, provided that Distributor pays Can-Fite [...] of Net Profits in the Territory and no other payments other than Transfer Price payments (to the extent Can-Fite is supplying the Authorized Generic) will be due to Can-Fite with respect to such sales.
3. MARKETING
3.1 Marketing Decisions.
Distributor shall control and make all decisions regarding the strategy and tactics of Marketing, selling and otherwise commercializing the Products for use in the Field in the Territory, including, without limitation, the methods of sale and distribution, organization and management of sales and Marketing, Packaging, pricing, and Labeling, appointment of SubDistributors, and other terms and conditions of such sales and Marketing. Notwithstanding the aforesaid, Distributor shall consult with Can-Fite prior to taking any material decision regarding the strategy and tactics of Marketing, selling and otherwise commercializing the Products in the Territory.
3.2 Marketing Plan.
Distributor will be responsible for assessing the market opportunities for the Product for use in the Field in the Territory and preparing and providing to Can-Fite, at least six (6) months prior to the First Commercial Sale, a marketing plan for the Product (“Marketing Plan”) which Marketing Plan shall set forth Distributor’s plan, strategy and proposed activities consistent with efforts appropriate for pharmaceuticals products of similar market potential to market the Product in the Territory. The Marketing Plan will include as appropriate without limitation, the following elements,
(a) a description of Distributor’s general strategy with respect to pre-launch and post-launch marketing, reimbursement strategies, advertising and promotion activities of the Product in the Territory;
| - 13 - |
(b) an estimated time schedule for the performance of the marketing activities;
(c) a description of the personnel resources of Distributor that will perform the marketing activities, including the number of sales representatives and physician calls; and
(d) a description of Distributor’s pricing strategy in the Territory.
Thereafter, Distributor shall, on or before November 1st in each Fiscal Year of the Term provide Can-Fite with a copy of Distributor’s Marketing Plan for the next Fiscal Year. Can-Fite may communicate comments to Distributor in respect of such Marketing Plans. Distributor agrees to consider such comments and shall provide a response to Can-Fite in respect of such comments, which response may include revisions to the Marketing Plan. Notwithstanding the foregoing, Distributor shall determine the Marketing Plan and will be responsible for its implementation and shall use Commercially Reasonable Efforts to achieve the objectives specified therein.
3.3 Advertising and Promotion.
Distributor shall provide to Can-Fite copies of the materials relating to the Marketing of the Supplied Products including print advertising and similar materials on a timely basis. All such materials shall comply in all material respects with applicable Laws and requirements of any applicable Regulatory Authority. Distributor shall not, in its Marketing materials, make any therapeutic claims or statements relating to the Supplied Products other than those authorized by the applicable Regulatory Authorities, and Distributor shall remain solely liable for all Marketing materials relating to the Supplied Products.
3.4 Information Sharing.
Can-Fite shall provide to Distributor in English such Product Information known to Can-Fite that may be useful or that Distributor requests or requires in preparing applications for or obtaining any Other Approvals and/or in the Marketing of the Product within the Territory, or in obtaining formulary listings or acceptance or approval of the Product by customers, potential customers, or buying agents or groups within the Territory. Distributor shall, and shall require its Affiliates and SubDistributors to, promptly provide to Can-Fite all Product Information that comes into its possession and all information relating to the Marketing or use of the Product.
3.5 Reports.
(a) Each Party shall promptly keep the other fully informed of all governmental and regulatory requirements, activities and plans of any Regulatory Authority including any changes thereto of which such Party becomes aware which materially affect, or are reasonably likely to materially affect, the sales or distribution of the Product in the Territory.
(b) After the First Commercial Sale of the Product, Distributor shall, throughout the Term, provide to Can-Fite on a calendar quarterly basis a statement containing the number of Units sold, the gross sales, the Net Sales and the Net Selling Price for each Product including details of all necessary calculations of the same, including the calculations which detail the differences between Net Sales and gross sales during such calendar quarter. Distributor shall provide such statement on a quarterly basis on or before the forty-fifth (45th) day following such calendar quarter.
| - 14 - |
(c) After the First Commercial Sale of the Product, Distributor shall on a calendar basis provide on or before the thirtieth (30th) day following each Fiscal Year, a report summarizing the status of Other Approvals and filings in terms of formulary listings and reimbursement pricing tier for each listing if applicable.
4. REGULATORY MATTERS AND PRODUCT DEVELOPMENT
4.1 Registration Responsibilities.
Distributor, provided that Can-Fite has, and continues to, comply with its covenants and obligations set forth in this Agreement, shall conduct and be responsible for:
(a) preparing the NDS or other applications, filing drug approval applications, including the NDS, answering any filing review issues and meeting with Regulatory Authorities;
(b) obtaining from the relevant Regulatory Authorities in the Territory, and for maintaining in force, all Regulatory Approvals in the Territory in Distributor’s name;
(c) the work for submitting variations to the Regulatory Approvals, the renewal of the Regulatory Approvals or any other regulatory procedure, answering any filing review issues and meeting with Regulatory Authorities; and
(d) paying all costs and expenses in connection with the costs of obtaining Regulatory Approval within the Territory.
Can-Fite acknowledges that Distributor, notwithstanding its efforts, does not guarantee that its efforts will result in the approval by Health Canada of the NDS or the issuance of any or all required Regulatory Approvals.
Can-Fite, upon written request of Distributor, shall provide Distributor, in English, with the Product Information and any requested or necessary documents relating to the Products (sufficient quantity of standard and working samples) and/or other information, for carrying out registration of the Products, making the NDS filing, and procuring the Regulatory Approvals in the Territory.
4.2 Development Responsibilities.
Can-Fite shall be responsible for all Product development activities including management of the clinical studies required in order to secure Regulatory Approval and shall use Commercially Reasonable Efforts in conducting such activities. Can-Fite agrees to include Canadian clinical sites in the pivotal phase III (or phase II/III, as applicable) Clinical Development program. Distributor shall not be responsible for any research and development costs associated with the Product. Distributor acknowledges that Can-Fite, notwithstanding its efforts, does not guarantee that its efforts will result in the success of any Clinical Development or that the Product will be found to be effective and be able to be Marketed and sold in the Territory for use in the Field.
| - 15 - |
4.3 Post-Approval Regulatory Responsibilities.
(a) Distributor shall be responsible for all pharmacovigilance activities related to the Supplied Product for use in the Field in the Territory.
(b) All substantive and material communications by Distributor with the Regulatory Authority for use in the Territory relating to the Supplied Product as Marketed for use in the Field in the Territory shall be promptly provided in writing to Can-Fite, and Distributor shall promptly provide to Can-Fite copies of all documents sent to or received from the Regulatory Authority regarding the Supplied Product.
(c) Distributor shall have the right, at its sole cost and expense, to conduct any post-regulatory approval, clinical Testing that Distributor chooses to conduct with respect to the Supplied Product that has received Regulatory Approval for use in the Field in the Territory, if any, for the continued and successful Marketing of the Supplied Product for use in the Field in the Territory. Notwithstanding the aforesaid, Distributor shall consult with Can-Fite prior to taking any material decision regarding the conduct of any such post regulatory approval or clinical Testing.
4.4 Other Approvals.
Distributor shall be responsible for all matters relating to the Other Approvals for the Product including filing the Product with, maintaining the Product on and dealing with, any federal, provincial or private formularies. The Distributor will apply for and will hold the Other Approvals in the Distributor’s name at all times for the benefit of Can-Fite. Distributor shall be responsible for all regulatory filings relating to the Product with the Other Authorities.
4.5 Monitoring ADE and Quality Complaint.
Distributor shall be responsible for receiving, monitoring, responding promptly to, tracking, or as may otherwise be required by applicable Law and Regulatory Authority, all Product-related inquiries, Product quality complaints, and ADE reports received by Distributor, its Affiliates or SubDistributors or by Can-Fite (and which Can-Fite shall have forwarded to Distributor) from individuals and/or health care professionals from within the Territory regarding the Supplied Product.
4.6 Quality and Technical Agreement.
The Parties shall negotiate in good faith and enter into a quality and technical agreement that appropriately addresses each Party’s responsibilities as they relate to Manufacturing, storage, distribution, regulatory, operational, Testing and quality issues regarding the Supplied Product no later than six (6) months prior to the First Commercial Sale (as such quality and technical agreement may be amended from time to time during the Term by mutual agreement of the Parties or to conform to requirements of applicable Law).
| - 16 - |
4.7 Pharmacovigilance Agreement.
The Parties shall negotiate in good faith and enter into a pharmacovigilance agreement that appropriately addresses each Party’s responsibilities as they relate to pharmacovigilance no later than six (6) months prior to the First Commercial Sale (as such pharmacovigilance agreement may be amended from time to time during the Term by mutual agreement of the Parties or to conform to requirements of applicable Law).
4.8 Cooperation.
Each of Can-Fite and Distributor shall provide to the other or if applicable, directly to the Authorities, any assistance and all documents reasonably necessary to enable the other to carry out its obligations under this Article 4. In general, requests for cooperation should be responded to by the other party within three (3) Business Days and both should make responsible efforts to ensure cooperation is maintained to ensure completion of the given project.
4.9 Joint Steering Committee.
A joint steering committee (“Joint Steering Committee”) will be established to monitor and supervise the progress of clinical studies and any other studies relating to development of the Product. The Joint Steering Committee will be composed of members as determined by Can-Fite, provided that the Joint Steering Committee includes one member from Distributor. A member determined by Can-Fite shall chair the Joint Steering Committee and in such capacity shall set its agenda and shall document the discussions held. The Joint Steering Committee will meet (in person or telephonically) as often as is reasonably necessary to accomplish its purpose but at least quarterly, on a mutually agreeable date and at a mutually agreed place. The Joint Steering Committee will make recommendations but will have no formal decision making authority as to the clinical studies. The Joint Steering Committee will dissolve once all Regulatory Approvals have been received for the Product.
5. ADES, PRODUCT QUALITY AND PRODUCT RECALLS
5.1 ADEs.
Each of Can-Fite and Distributor and their respective Affiliates, SubDistributors, Approved Manufacturers, agents or other relevant parties shall inform the other of all known or suspected ADE’s associated with Supplied Product, of which it is notified, or otherwise becomes aware, as soon as reasonably possible but in any event within ten (10) days for ADEs and forty-eight (48) hours for Serious ADEs or within any time limits required by applicable Law, whichever is shorter. “Serious ADEs” means, with respect to a serious adverse event or reaction, is any noxious and unintended response to a drug that at any dose:
| ● | requires in-patient hospitalization or prolongation of existing hospitalization; |
| - 17 - |
| ● | causes congenital malformation; |
| ● | results in persistent or significant disability or incapacity; |
| ● | is a congenital anomaly/birth defect; |
| ● | is a medically important event or reaction; |
| ● | results in death; or |
| ● | is life-threatening. |
5.2 Product Complaints other than ADEs.
(a) Each Party shall submit to the other Party, within three (3) Business Days of receipt any complaints or issues that question Supplied Product quality (other than ADEs or performance of the Supplied Product) received by that Party or any of its Affiliates or, in the case of Distributor, its SubDistributors, to which that Party must respond, together with all evidence then available and all other information relating thereto subsequently obtained or produced by either Party.
(b) Can-Fite shall respond, in writing (including by email) or by telephone, to inquiries made by Distributor relating to the Manufacturing or Packaging of Supplied Product as promptly as practicable, but in no event, later than fifteen (15) Business Days of receipt of the such inquiry, with such information as Distributor may reasonably require addressing the inquiry.
(c) Each of Distributor and Can-Fite shall promptly notify the other of any notice of non-compliance with any Laws applicable to Supplied Product or the Packaging of Supplied Product, received from any Authority having jurisdiction in the Territory, and of any request for or initiation of any inspection of any facility of either Can-Fite or Distributor, or any Affiliate of Can-Fite or Distributor, or any Approved Manufacturer, or Contract Finisher that Manufactures, Packages, Tests or stores any Supplied Product.
(d) Each Party shall inform the other of all known or suspected adverse drug reactions associated with the Product (not otherwise covered above), of which it is notified, or otherwise becomes aware, within two (2) weeks, together with all evidence then available and all other information relating thereto subsequently obtained or produced by either Party.
5.3 Product Recall.
(a) Distributor will maintain or cause to be maintained such traceability records as are necessary to permit a recall, market withdrawal or field correction of the Supplied Product including inventory withdrawal in connection with any of the foregoing (each a “Recall”).
(b) Each Party shall promptly (but in any case, not later than twenty-four (24) hours of receipt) notify the other Party in writing of any information which indicates a Recall of any Supplied Product may be necessary, any safety or regulatory concerns, or any order, request or directive of a court or other Regulatory Authority requesting or requiring a Recall.
| - 18 - |
(c) To the extent permitted by circumstances, the Parties will confer before initiating any Recall. If the Parties do not agree on the need for or the extent of such a Recall, either Party may authorize the Recall.
(d) Distributor shall be responsible for the carrying out of any and all Recalls with respect to the Supplied Product in accordance with applicable Laws.
(e) If any Recall is required primarily and substantially because of (i) breach of Can-Fite of a representation, warranty or covenant hereunder, or (ii) failure of the Supplied Product to conform to the Specifications at the time title is transferred to the Distributor, as confirmed by a mutually acceptable Third Party Laboratory, including a Latent Defect that is shown to have existed at the time of such title transfer, Can-Fite will be responsible for the direct costs of such Recall, will reimburse Distributor, its Affiliates, and SubDistributors for all of their direct out-of-pocket costs and direct expenses related to such Recall. The Parties shall promptly discuss whether to credit or refund the Transfer Price of any Supplied Product subject to any Recall and, if the Parties are unable to agree, then Can-Fite shall supply to Distributor free of cost and expense replacement Supplied Product and Distributor will distribute the replacement Supplied Product.
(f) If any Recall is required primarily or substantially in circumstances caused by the negligence, mistake, fault, error or omission of Distributor, its Affiliates or subcontractors, including any breach by Distributor of a representation, warranty or covenant hereunder, Distributor will be responsible for the direct costs of such Recall and will reimburse Can-Fite and its Affiliates for all of their direct out-of-pocket costs and direct expenses related to such Recall.
(g) If any Recall is required under circumstances not covered in Clauses (e) or (f) above, the parties will equally share the direct costs of such Recall, including direct out-of-pocket costs and expenses related to such Recall.
5.4 Cooperation as to ADE, Product Inquiries and Recalls.
Each of Can-Fite and Distributor shall provide to each other in a timely manner all information which the other Party reasonably requests regarding Supplied Product in order to enable the other Party to comply with all Laws applicable to Supplied Product in the Territory and in order to enable Can-Fite to comply with all Laws applicable to the Product outside the Territory. Without limiting the foregoing, each Party will cooperate fully with the other Party in connection with any Recall efforts.
| - 19 - |
6. PURCHASE PRICE AND SUPPLY OF PRODUCTS
6.1 Supply of Products.
(a) Can-Fite will be responsible for the Manufacture of Supplied Product and shall cause its Approved Manufacturer to Manufacture and, if applicable, its Contract Finisher to Package and label the Supplied Product for the Distributor. Except as provided in Section 6.3, the Distributor shall purchase from Can-Fite all of the Distributor’s requirements for the Supplied Product for use in the Field in the Territory during the Term, pursuant to purchase orders submitted by the Distributor or its Affiliates to Can-Fite from time to time in accordance with Section 6.2.
(b) Except as provided in Section 6.3, Can-Fite shall supply or cause the Approved Manufacturer to supply all Supplied Product for use in the Field for distribution in the Territory solely to Distributor during the Term in accordance with the terms and conditions of this Agreement.
(c) Can-Fite and its Approved Manufacturer and if applicable its Contract Packager shall be responsible for the purchase of adequate supplies of all materials, including, without limitation, raw materials, in accordance with the NDS and other filings with Regulatory Authorities for the Supplied Product as necessary to supply finished Supplied Product to the Distributor in accordance with the Specifications and applicable Law.
(d) The Supplied Product shall be manufactured with labeling and packaging as specified by Distributor and in accordance with applicable Laws. At least four (4) months prior to the First Commercial Sale with respect of each Supplied Product, Distributor shall, at its sole cost and expense, provide Can-Fite with final specifications for such labeling and packaging for Supplied Product, including all necessary photo-ready artwork (or its substantial equivalent). Distributor, from time to time may update the labeling and packaging specifications. Such updates shall be subject to the prior written approval of Can-Fite, not to be unreasonably withheld, delayed or conditioned. Distributor shall, at Distributor’s expense, use Commercially Reasonable Efforts to secure any approvals required by Health Canada or any other applicable Regulatory Authority to effect such revisions to the labeling and packaging.
(e) The terms and conditions of this Agreement shall control the Manufacture and supply of Supplied Product by Can-Fite to Distributor, and no terms or conditions contained in any purchase order, acknowledgment, invoice, bill of lading, acceptance or other pre-printed form issued by any Party shall have any force or effect to the extent they are inconsistent with or modify the terms and conditions of this Agreement including those set forth in this Section 6.1.
(f) Out-of-pocket costs associated with regulatory changes requested by (i) Health Canada which cause finished product, raw materials, labeling and other materials to be discarded will be shared equally between Distributor and Can-Fite, and (ii) Distributor which cause finished product, raw materials, labeling and other materials to be discarded will be borne by Distributor.
| - 20 - |
(g) The costs of implementing, chemistry, manufacturing and controls changes or ancillary additional testing not included in the New Drug Submission that is requested after Regulatory Approval, validation and launch, shall be shared equally between Distributor and Can-Fite if requested by a Regulatory Authority and shall be borne one hundred percent (100%) by Distributor, if requested by Distributor.
6.2 Forecasts, Orders.
(a) Forecasts; Firm Orders.
(i) Distributor shall submit to Can-Fite, at least four (4) months prior to the estimated First Commercial Sale, a written forecast for the first twelve (12) month period of the quantity of Supplied Product (a “Forecast”) that Distributor desires to have delivered to it for Product launch purposes. The Parties agree that the Supplied Product quantities specified in Distributor’s initial Firm Order, represent Distributor’s launch quantities of the Supplied Product. Thereafter, on or before the tenth (10) calendar day of each month during the Term, Distributor shall provide a written, updated consecutive twelve (12) month Forecast (on 10th of January, the Forecast will be until the 10th of January of the next year, on 10 of February, the Forecast will be until the 10 of February of the next year, etc) of the Supplied Product, including the expected purchase order dates and shipping dates for each order during the following twelve (12) consecutive calendar month period beginning on the first day of the following calendar month. Can-Fite acknowledges that such Forecasts are only estimates of Distributor’s purchase order requirements of the Supplied Product and that Distributor shall not be bound by any such estimate, except that beginning after the First Commercial Sale (A) the portion of the Forecast commencing on the first day of the Forecast period and ending on the last day of the third full calendar month after the first day of the Forecast period shall be deemed a firm order period for which Distributor is obligated to issue purchase orders and take ownership of Supplied Product requirements (each, a “Firm Order”) and (B) the first two months of each Forecast will repeat the balance of the Firm Order period of the prior Forecast, and the first three months of the Forecast shall constitute the new Firm Order period for which Distributor is obligated to purchase and take delivery of the forecasted Supplied Product, and (C) the third month of the Firm Order period may vary by up to twenty percent (20%) from that set forth on the fourth month of the prior Forecast.
(ii) Can-Fite shall have no liability to Distributor for any failure or inability to supply Distributor in the third month of the Firm Order, with quantities of Product in excess of amounts described in Section 6.2(a)(i)(C).
(iii) Can-Fite shall notify Distributor if Can-Fite determines that it will be unable to meet the quantities of Supplied Product in excess of Can-Fite’s obligations as contemplated in Section 6.2(a)(ii), as soon as practicable but in any event within ten (10) days after receiving the applicable purchase orders from Distributor.
| - 21 - |
(b) Purchase Orders.
(i) Distributor shall deliver to Can-Fite its initial purchase order for the Product within one hundred and twenty (120) days prior to the shipping date required by Distributor. The initial purchase order for the Product shall be for sufficient quantities to satisfy sales requirements of Distributor for no less than the first three (3) months of sales of Product. The purchase order shall specify the location to which Product is to be shipped (which is not the same as where title passes under Section 6.4) and the date by which Product must be shipped to such location.
(ii) During the Term, Distributor shall submit to Can-Fite, purchase orders for the last month of each Firm Order period no later than one hundred and five (105) days (“Deadline Date”) prior to the required shipping date, identifying the quantities of Supplied Product. The purchase order shall also specify the location to which Product is to be shipped (which is not the same as where title passes under Section 6.4) and the date by which the Product must be shipped to such location. Such purchase orders shall comply with the Firm Order period provisions set out in Section 6.2(a). If a purchase order for any month is not submitted by the Deadline Date, Distributor shall be deemed to have submitted a purchase order for such month for the amount of Supplied Product set forth in Distributor’s most recent Forecast for such month.
(iii) In the event that a purchase order requires an amount higher than one hundred and twenty percent (120%) of the amount set forth in the Forecast for a given month (the “Additional Quantity”), Can-Fite shall either (i) confirm to Distributor its acceptance of such purchase order with respect to the Additional Quantity within ten (10) calendar days of receipt of such purchase order or (ii) in the event that Can-Fite cannot supply the Additional Quantity indicated in such purchase order, Can-Fite shall provide Distributor within such ten (10) day period with a delivery schedule for such Additional Quantity which Can-Fite will commit to meet.
(c) Batch Sizes. Once the validation batch inventories have been depleted, Forecasts and purchase orders shall be in minimum batch sizes which are commercially reasonably under the specific circumstances of this Agreement as determined by the parties jointly in good faith.
(d) Satisfaction by Can-Fite Affiliates and Approved Manufacturers. Can-Fite may cause any Affiliate or its Approved Manufacturer to satisfy any of the obligations of Can-Fite under this Article 6. Notwithstanding the previous sentence, Can-Fite shall remain fully responsible and liable to Distributor for the performance of all terms of this Article 6 by its Affiliates or Approved Manufacturers.
(e) Alternative Delivery of Forecasts and Payments. Can-Fite may direct Distributor, in writing, to deliver its Forecasts, purchase orders and payments to an Affiliate of Can-Fite or an Approved Manufacturer, with a copy to Can-Fite, and to receive shipments of a Supplied Product from that Affiliate or Approved Manufacturer.
(f) Form of Purchase Orders. All purchase orders placed by Distributor hereunder shall be in a form reasonably acceptable to Can-Fite, and Distributor shall send such purchase orders by email, courier or mutually agreed upon method. Except for terms relating only to quantities, shipping dates and delivery destinations, none of the terms and conditions contained in any purchase order, invoice or similar documents shall have any effect upon or change the provisions of this Agreement unless signed by both Parties and specifically stating that the Parties intend to vary the terms hereof.
| - 22 - |
6.3 Continuity of Supply.
(a) A “Supply Interruption” shall have occurred in the event that (i) Can-Fite is unable to supply any Supplied Product to Distributor pursuant to Section 6.2 for sixty (60) days or more of the anticipated date of delivery specified in a purchase order, to the location specified therein, or (ii) Can-Fite is unable to deliver on a timely basis at least eighty-five percent (85%) of the amount covered by Purchase Orders issued by Distributor pursuant to Sections 6.2(b) for four (4) or more consecutive months, (whether as a result of a Force Majeure event, GMP issues, failure to meet quality standards, or otherwise). In the event that circumstances arise that may give rise to a potential Supply Interruption, the Parties will work collaboratively in good faith to avoid a Supply Interruption and in such case Can-Fite agrees to use Commercially Reasonable Efforts to provide Distributor with the same or greater percentage of Supplied Product for its Firm Orders, as the percentage of Supplied Product it provides to any other distributor of Product outside the Territory with respect to its Firm Orders; provided, however, that the foregoing shall not lessen or adversely impact Distributor’s rights under this Section 6.3. Without limiting the foregoing, Can-Fite’s Commercially Reasonable Efforts shall include without limitation sourcing Product from Alternate Suppliers, and Can-Fite shall supply or shall cause such Alternate Supplier to sell Product to Distributor, on the same terms and conditions as Distributor was otherwise purchasing Supplied Product from Can-Fite hereunder, provided that Distributor shall approve, such approval not to be unreasonably withheld, any Alternate Supplier prior to purchasing any Product manufactured by such Alternate Supplier.
(b) Can-Fite shall have six (6) months from the date of the occurrence of the Supply Interruption to resume compliance with its supply obligations in accordance with this Agreement. Can-Fite shall provide Distributor with written notice of its ability to resume supply (the “Resumption Notice”), if Can-Fite is able to resume supply within such six (6) month period. The Resumption Notice must: (i) list the date on which Can-Fite will be able to resume its supply obligations (which must not be later than one month from the date of the notice; and (ii) include a statement of Can-Fite’s ability to resume such obligations by that date which describes in reasonable detail what problems have been rectified and include a representation (which will be deemed a Can-Fite representation under this Agreement) that Can-Fite is able to fulfill its supply obligations under this Agreement.
(c) If a Supply Interruption occurs and Can-Fite is not able to resume supply within the six (6) month period provided in Section 6.3(b), then Distributor will have the right, but not the obligation, in its sole discretion upon written notice to Can-Fite, to terminate this Agreement.
| - 23 - |
6.4 Method of Delivery of Supplied Product.
Can-Fite shall notify Distributor of, as applicable, the location of the Approved Manufacturer or Contract Finisher and of any change thereto. Distributor shall advise Can-Fite in writing at least fifteen (15) days in advance of the scheduled shipping date specified in the applicable purchase order of the carrier to be used to ship Supplied Product to Distributor. Distributor will cause such carrier to comply with all applicable Laws for the shipment of Supplied Product. Can-Fite shall determine the appropriate carrier if Can-Fite receives no direction from Distributor at least fifteen (15) days in advance of the scheduled shipping date specified in the applicable purchase order to use a particular carrier. Can-Fite shall deliver all quantities of Supplied Product to Distributor FCA (Incoterms 2010) the manufacturing facility or warehouse of Distributor, its Approved Manufacturer or Contract Finisher and risk of loss and title to Supplied Product shall pass to Distributor immediately upon the loading of Supplied Product at such facility or warehouse. Distributor shall be responsible for all freight, insurance, handling, fees, taxes and other costs associated with shipment or importation of Supplied Product.
6.5 Acceptance, Rejection and Revocation of Acceptance.
(a) Can-Fite shall provide a certificate of analysis and other documents (collectively, the “COA”) in such forms as the Parties shall mutually agree upon, for any Supplied Product batch delivered to Distributor hereunder certifying that such Supplied Products have been Manufactured and Packaged in compliance with the Specifications, GMPs and all other applicable Regulatory Requirements and with an expiry date of not less than thirty (30) months from the date of shipment.
(b) Distributor shall inspect or shall cause to be inspected all shipments of Supplied Product promptly upon receipt. Distributor may reject any Supplied Product which does not conform to the Specifications at the time of receipt at Distributor’s location. Distributor shall make any such rejection in writing, within thirty (30) days of the later of the receipt of the COA or the Supplied Product at the facility designated by Distributor in the applicable Purchase Order (the “Stipulated Rejection Period”), to Can-Fite, and shall indicate the reasons for such rejection (the “Rejection Notice”).
(c) If Distributor has not delivered a Rejection Notice within the Stipulated Rejection Period, Distributor shall be deemed to have accepted that shipment of Supplied Product. Once Distributor has accepted or has been deemed to have accepted a shipment of Supplied Product, and except with respect to Latent Defects discovered by Distributor or Distributor’s customers after the expiration of the Stipulated Rejection Period, Distributor may not exercise any rights to subsequently reject such shipment under this Section 6.5.
6.6 Rejection Procedures.
(a) After Can-Fite receives the Rejection Notice, it will evaluate process issues and the reasons given by Distributor for the Rejection. Can-Fite shall use good faith efforts to promptly notify Distributor whether it agrees with the basis for Distributor’s rejection, but in no event shall such notice be given later than thirty (30) days of Can-Fite’s receipt of a Rejection Notice. If Can-Fite does not so notify Distributor within thirty (30) days of receipt of the Rejection Notice as to whether it agrees with the basis of Distributor’s rejection, Can-Fite shall be deemed to be in agreement therewith.
| - 24 - |
(b) If Can-Fite agrees with or is deemed to agree with the basis for Distributor’s rejection, Can-Fite shall promptly replace, at no cost to the Distributor, such rejected Supplied Product.
(c) If Can-Fite disagrees with the basis for Distributor’s rejection specified in the Rejection Notice, Can-Fite shall promptly replace such rejected Supplied Product. No payment shall be due with respect to the replacement Product until it is determined which Party shall bear the burden of such cost hereunder. The Parties shall submit samples of the rejected Supplied Product to the Third Party Laboratory, which shall determine whether such Supplied Product meets the Specifications and, as part of this process, may also carry out a full investigation of the Manufacturing process (including, as necessary, the Approved Manufacturing Site) for such Supplied Product if it reasonably believes such an investigation is necessary to resolve the disagreement. The Parties agree that the determination of the Third Party Laboratory, after it has assessed the retention samples and following any full investigation of the Manufacturing process it conducts, shall be final and determinative. If the Third Party Laboratory determines that the retained samples meet the Specifications, the rejection by Distributor is deemed to be unjustified, and Distributor shall promptly pay Can-Fite for any replacement Product. If the Third Party Laboratory determines that the relevant shipment of Supplied Product does not meet the Specifications, Can-Fite shall not invoice Distributor for the replacement Supplied Product. The Party against whom the Third Party Laboratory rules shall also bear the fees charged by the Third Party Laboratory in connection with resolution of the disagreement, including all out-of-pocket costs of investigating the Manufacturing process.
(d) At Can-Fite’s election and upon authorization from Can-Fite, Distributor shall destroy the rejected Supplied Product promptly and provide Can-Fite with certification of such destruction unless Can-Fite elects to have the Supplied Product returned, in which event Distributor shall cooperate in arranging such return. If Can-Fite agrees with the basis for Distributor’s rejection or if the Third Party Laboratory rules against Can-Fite, Can-Fite shall pay the cost of destroying or returning the Supplied Product. In all other cases, Distributor shall bear such costs.
(e) Notwithstanding any of the other provisions in this Agreement and without limiting any other provision herein, Distributor agrees that the remedies set forth in this Section 6.6 are Distributor’s sole and exclusive remedies with respect to the rejection of Supplied Product.
6.7 Prices and Payments.
(a) Subject to the provisions of Section 6.3 hereof, Distributor shall pay to Can-Fite or Can-Fite’s designees the following:
(i) The Milestone Payments in the amounts and at the time as set out in Part A of Schedule C;
(ii) The Transfer Price for Supplied Product supplied by Can-Fite in the amounts calculated in accordance with the provisions of Part B of Schedule C;
| - 25 - |
(iii) Royalty payments calculated in accordance with the provisions of Part C of Schedule C; and
(iv) The share of the Net Profits from Distributor’s sale of an Authorized Generic in accordance with Section 2.4.
(b) Distributor shall pay all insurance and shipping costs and any Taxes imposed on the importation of Supplied Product into the Territory.
(c) Distributor shall be responsible for the payment of any duties, levies or Taxes applied to the sale of Supplied Product in the Territory by any relevant Tax authority.
(d) Any payments to be made hereunder and which have not been made by the due date, shall accrue interest at any monthly rate equal to [...]. Additionally, Distributor shall be responsible for all reasonable attorneys’ fees, witness fees and court costs and other costs incurred by Can-Fite to recover amounts owing to it hereunder.
(e) Distributor shall make all payments contemplated by this Agreement in the lawful currency of Canada and Distributor shall make such payments to such address as Can-Fite may from time to time direct in writing to Distributor.
6.8 Audit.
Distributor shall keep and retain complete and accurate records pertaining to the disposition of Supplied Product and amounts payable under this Agreement (including, without limitations, amounts payable pursuant to Section 2.4 hereof) for each Fiscal Year or part thereof during the Term in sufficient detail to permit Can-Fite to confirm the accuracy of all payments made or due hereunder for a period of three (3) years following the applicable Fiscal Year or part thereof. At Can-Fite’s request, Can-Fite and Distributor shall jointly appoint an independent internationally recognized audit firm to audit the books of account of Distributor in order to determine whether Distributor has properly reported and accounted for any fees or payments due to Can-Fite pursuant to this Agreement. The appointed audit firm may perform audits during regular business hours, not more than once in any calendar year during the Term and upon reasonable prior notice to Distributor. Can-Fite shall bear the audit fees unless such audit firm determines that the amount actually due Can-Fite, in the aggregate, exceeds the amounts paid or deemed paid by Distributor hereunder by [...], in which case Distributor shall bear the audit fees. Distributor shall forthwith pay any amounts discovered to be due pursuant to an audit together with interest from the date payment was originally due at a monthly rate equal to [...]. The results of the audit shall be final and binding upon the Parties.
| - 26 - |
6.9 Facility Audits.
(a) Distributor and/or its nominee shall have the right to conduct an audit of any Approved Manufacturing Site(s) at which the Supplied Product is being Manufactured, of Manufacturing records relating to the production of such Product, if applicable, of the Contract Finisher(s)’ facility where Supplied Product is Packaged and of any correspondence between Can-Fite and the Regulatory Authority related to such Supplied Product or such facilities, during business hours upon ten (10) Business Days prior written notice to Can-Fite not more than once per Fiscal Year during the Term of this Agreement, unless either Party, any Authority or any Third Party raises any questions about the quality of the Supplied Product which could have a material detrimental effect on the sales or use of Supplied Product, in which case Distributor’s audit right shall not be subject to the foregoing limitation until the specific issue in question has been resolved, and Can-Fite shall promptly supply or cause its Approved Manufacturer to supply to Distributor all data and results relating to all Testing performed in connection with the issue in question.
(b) Can-Fite and/or its nominee shall have the right to conduct an audit of the facilities and records of Distributor and/or its SubDistributors and/or their Affiliates relating to the Marketing, Testing, and storage of Supplied Product and of any correspondence between Distributor and/or its SubDistributors and/or their Affiliates and the Regulatory Authority related to Supplied Product or such facilities, during business hours upon ten (10) Business Days prior written notice to Distributor not more than once per Fiscal Year during the Term of this Agreement, unless any Authority or any Third Party raises any questions about the quality of the Supplied Product or the Testing and Marketing thereof which could have a material detrimental effect on the sales or use of Supplied Product, in which case Can-Fite’s audit right shall not be subject to the foregoing annual limitation until the specific issue or question has been resolved, and Distributor shall promptly supply to Can-Fite all data and results relating to all Testing performed by Distributor on Supplied Product.
7. INTELLECTUAL PROPERTY
7.1 Ownership of Can-Fite Intellectual Property.
Can-Fite shall retain all of its rights, title and interest in and to all Product Technology, Can-Fite Trademarks, copyrights, and all other industrial and Intellectual Property embodied in or which covers the Product, in each case which is owned, held, or licensed by it as of the Effective Date or thereafter or developed, created or discovered by it or on its behalf during the Term, subject to the rights granted in this Agreement. Except as otherwise expressly provided in this Agreement, Distributor has and shall have no right, title or interest in any Intellectual Property owned by or licensed to Can-Fite relating to the Product including the Product Technology and Can-Fite Trademarks.
7.2 Ownership of Distributor Intellectual Property.
Distributor shall retain all of its right, title and interest in and to all copyrights and all other Intellectual Property owned, held, or licensed by it as of the Effective Date or thereafter developed, created or discovered by it or on its behalf during the Term, including Trademarks. For clarification purposes, the Parties agree that nothing herein grants, or constitutes an agreement or obligation to grant, to Can-Fite, any Approved Manufacturer or any of their Affiliates or other Third Party any right, title or interest in, to or under any of the Trademarks.
| - 27 - |
7.3 Maintenance and Prosecution of Product Patents.
As between Distributor and Can-Fite, Can-Fite shall remain responsible for maintenance of Product Patents and shall bear all expenses associated therewith including prosecution, renewal and other fees necessary to obtain and maintain the Product Patents in full force and effect until the earlier of their expiration or the termination or expiration of this Agreement.
7.4 Notice of Patent Infringement.
(a) Information Concerning Infringement. If either Party shall learn of (i) any claim or assertion that the Manufacture, Marketing, Packaging or Testing of the Supplied Product, or the use of the Product Technology or other Intellectual Property related to the Supplied Product infringes, misappropriates or otherwise violates the Intellectual Property rights of any Third Party, or (ii) the actual or threatened infringement, misappropriation or other violation by any Third Party of the Product Technology or other Intellectual Property related to the Product, then the Party becoming so informed shall as soon as reasonably practicable, but in all events within ten (10) Business Days thereof, notify the other Party of such claim or assertion, or actual or threatened infringement, misappropriation or other violation.
(b) Potential Infringement. In the event either Can-Fite or Distributor learns of any Third Party patents which may cover the Manufacturing, Marketing, Testing or Packaging of the Supplied Product in the Territory, such Party will promptly notify the other Party. The Parties agree to confer in good faith regarding such potential infringement risk and to explore reasonable alternatives for avoiding such risk and to provide such information to each other as either Party may reasonably request.
(c) Third Party Claims; Defense by Can-Fite. If a Third Party files or threatens to file a claim, suit or action against Can-Fite or Distributor claiming that a patent or other Intellectual Property right held by or licensed to it is infringed, misappropriated or otherwise violated by the Manufacturing, Marketing or Testing or Packaging of the Supplied Product, and such claim, suit or action arises out of Distributor’s exercise of its rights under this Agreement, the Parties shall confer in good faith regarding such alleged infringement, misappropriation or other violation. Can-Fite may, but shall not be obligated to, defend any such claims, suits or actions and shall notify Distributor of its election within thirty (30) days of receipt of notice. If Can-Fite elects to defend such claims, suits or actions, it shall notify Distributor that it has elected to do so. Can-Fite shall provide information to Distributor of the conduct of the defense of such claims, suits or actions as Distributor may reasonably request from time to time. Distributor will assist in the defense of any such claim, suit or action as reasonably requested by Can-Fite. Can-Fite and Distributor shall be equally responsible for and pay any payments made to Third Parties, and shall share equally all fees, costs and expenses (including, without limitation, outside attorneys’ fees and expenses of Distributor and Can-Fite) as a result of any actual or alleged infringement. Can-Fite shall not settle any such claim, suit or action if such settlement would impose on Distributor the obligation to pay or contribute to any claim, without the prior express written consent of Distributor, which shall not be unreasonably withheld, conditioned, or delayed. The Parties agree that in the event Can-Fite fails to remit any payments required to be made to Third Parties or related expenses as provided above, and Distributor, in its sole discretion, elects to make such payments, Distributor may set off such amounts against the payments to be made by Distributor to Can-Fite pursuant to Section 6.7(a).
| - 28 - |
(d) Defense by Distributor. If Can-Fite elects not to defend against Third Party claims, suits or actions pursuant to Section 7.4(c), Can-Fite shall give notice of its decision to Distributor within reasonably sufficient time to permit Distributor, at its option and without requirement, to defend against such claims, suits or actions. If Distributor, in its sole discretion, elects to defend such Third Party claims, suits or actions, it shall notify Can-Fite that it has elected to do so and shall notify Can-Fite in writing of its proposed legal counsel. Distributor shall provide information to Can-Fite of the conduct of the defense of such claims, suits or actions as Can-Fite may reasonably request from time to time and Can-Fite shall assist in such defense as reasonably requested by Distributor. Can-Fite and Distributor shall be equally responsible for and pay any payments made to Third Parties and shall share equally all fees, costs and expenses (including, without limitation, outside attorneys’ fees of Distributor and Can-Fite) as a result of any actual or alleged infringement. The Parties agree that in the event Can-Fite fails to remit any payments required to be made to Third Parties or related expenses, Distributor may set off such amounts against the payments to be made by Distributor to Can-Fite pursuant to Section 6.7(a). Distributor shall not settle any such claim, suit or action if such settlement would impose on Can-Fite the obligation to pay any claim, without the prior express written consent of Can-Fite, which shall not be unreasonably withheld, conditioned, or delayed.
7.5 Can-Fite Trademarks Indemnified Infringement Claims.
Can-Fite agrees that it shall, at its own cost and expense, defend, indemnify and hold harmless the Distributor Indemnitees from and against any and all liabilities, losses, damages, actions, claims and expenses suffered or incurred by Distributor Indemnitees (including reasonable attorneys’ fees, court costs and expert witnesses’ fees) (collectively “Claims”) arising out of, resulting from or otherwise related to the Can-Fite Trademarks used by any Distributor Indemnitee as permitted by this Agreement, provided that any such Claim does not arise from Distributor’s breach of this Agreement, or arise from Distributor Indemnitee’s negligent or intentionally wrongful conduct. The Parties agree that nothing in this Section 7.5 limits Can-Fite’s obligations to bear an equal share of any payments made to Third Parties as provided under Section 7.4(c) or Section 7.4(d) or indemnity obligations under Article 10 of this Agreement).
7.6 Trademarks Indemnified Infringement Claims.
Distributor shall, at its own cost and expense, defend, indemnify and hold harmless the Can-Fite Indemnitees from and against any and all Claims suffered or incurred by Can-Fite Indemnitees (including reasonable attorneys’ fees, court costs and expert witnesses’ fees) arising out of, resulting from or otherwise related to the Trademarks used by Distributor, its Affiliates, sublicensees, SubDistributors or agents, other than the Can-Fite Trademarks, provided that any such Claim does not arise from Can-Fite’s breach of this Agreement, or arise from Can-Fite Indemnitee’s negligent or intentionally wrongful conduct. The Parties agree that nothing in this Section 7.6 limits Distributor’s obligations to bear an equal share of any payments made to Third Parties as provided under Section 7.4(c) or Section 7.4(d) or indemnity obligations under Article 10 of this Agreement).
| - 29 - |
7.7 Infringement of Product Technology by a Third Party.
(a) In the event that any Party becomes aware of any Person infringing or potentially infringing the Product Technology in the Territory, whether by direct or indirect infringement, or by misappropriation of Product Technology, it shall promptly notify the other Party. Distributor shall not give notice (written or other) of infringement of any of the Product Technology or infringement or misappropriation of other Intellectual Property of Can-Fite to any Third Party without Can-Fite’s prior written consent; provided, however, that Distributor may give such notice to a Third Party without Can-Fite’s consent but upon thirty (30) days prior written notice to Can-Fite, if (a) Can-Fite has declined to take steps to abate such infringement or misappropriation and Distributor has the right to enforce the Product Technology or other Intellectual Property of Can-Fite against such Third Party infringer as set forth in this Article 7; or (b) disclosure is required by applicable Laws to which Distributor is subject. In a case where Distributor receives a notice of allegation under the Patented Medicines (Notice of Compliance) Regulations in respect of the Product Patents, Can-Fite shall use Commercially Reasonable Efforts (including financial assistance) to assist Distributor in seeking an order, within the forty-five (45) day deadline required under the Patented Medicines (Notice of Compliance) Regulations, prohibiting Health Canada from issuing a notice of compliance for a Third Party’s drug product until the expiration of the Product Patents that are the subject of such notice of allegation. For any actions other than those under the Patented Medicines (Notice of Compliance) Regulations, unless the Parties otherwise mutually agree in writing, Can-Fite shall have the initial right, but not the obligation to enforce the Product Technology or defend any declaratory action with respect thereto (an “Enforcement Action”), at its expense and using its Commercially Reasonable Efforts, and Distributor shall give all reasonable assistance (excluding financial assistance) to Can-Fite in such Enforcement Action. However, if (i) Can-Fite agrees in writing not to bring or defend an Enforcement Action with respect to any Product Technology in the Territory or (ii) within ninety (90) days following a written request by Distributor to do so and confirmation of facts reasonably supporting existence of such actual or suspected infringement with respect to Product Technology in the Territory for which Distributor has license rights under this Agreement, Can-Fite fails to bring or defend an Enforcement Action or take other commercially reasonable action to protect the Product Technology from such infringement, or to abate such infringement, then Distributor shall have the right, but not the obligation, at its sole discretion, to institute an Enforcement Action in its own name at its own expense, and with the right to control the course of such Enforcement Action (and Can-Fite shall provide all reasonable assistance to Distributor for such Enforcement Action, at Distributor’s expense, including joining such Enforcement Action if necessary to maintain the Enforcement Action, and Can-Fite shall have the right to join and participate in the Enforcement Action whether or not such joinder is requested by Distributor; if not requested by Distributor, such participation will be at Can-Fite’s expense). Any amounts recovered with respect to any Enforcement Action pursuant to this Section 7.7(a) shall be applied first to reimburse the Parties for their reasonable out-of-pocket expenses (including reasonable attorneys’ fees) in the prosecution of such infringement. The remainder shall be deemed Net Sales. Notwithstanding any provision to the contrary, Distributor shall not be precluded from seeking, in its own independent cause of action, the recovery of damages resulting from its own lost sales, price erosions or similar damages suffered or incurred by Distributor.
| - 30 - |
7.8 Trademarks.
(a) License. Subject to the terms and conditions of this Agreement, Can-Fite hereby grants to Distributor an exclusive right for use in the Field within the Territory to use the Can-Fite Trademarks solely for the purposes of Marketing the Products under this Agreement. Such license shall not preclude Can-Fite and/or any Approved Manufacturer from using Can-Fite Trademarks within the Territory for any Product that is to be Marketed or sold outside the Territory.
(b) No Obligation to Use Trademarks. Distributor may use the Can-Fite Trademarks solely for the Marketing of the Products for use in the Field in the Territory. Distributor may use its own Trademarks on Product Packaging, brochures and other promotional materials to identify itself as the distributor of the Product provided that Distributor has obtained the applicable Regulatory Approval.
(c) Can-Fite Ownership. Distributor hereby agrees to and recognizes Can-Fite’s exclusive ownership or license rights of the Can-Fite Trademarks and the renown of the Can-Fite Trademarks. Distributor agrees not to take any action inconsistent with such ownership and further agrees to take any action, at Can-Fite’s expense, which Can-Fite reasonably deems necessary to establish and preserve Can-Fite’s exclusive rights in and to the Can-Fite Trademarks including cooperating in the registration of the Can-Fite Trademarks on the trademark registry or other appropriate registration procedure in each jurisdiction in the Territory.
(d) Distributor Ownership. Can-Fite hereby agrees to and recognizes Distributor’s exclusive ownership or license rights of the Trademarks and the renown of the Trademarks. Can-Fite agrees not to take any action inconsistent with such ownership and further agrees to take any action, at Distributor’s expense, which Distributor reasonably deems necessary to establish and preserve Distributor’s exclusive rights in and to the Trademarks including cooperating in the registration of the Trademarks on the trademark registry or other appropriate registration procedure in each jurisdiction in the Territory.
(e) Can-Fite Goodwill. The Parties agree that all use of the Can-Fite Trademarks by Distributor shall be for the sole and exclusive benefit of Can-Fite and the goodwill and reputation accrued in connection with Distributor’s or its Affiliates’ or SubDistributors’ use of the Can-Fite Trademarks shall accrue solely to Can-Fite. In the event Distributor acquires any right, title or interest in or to or relating to the Can-Fite Trademarks for any reason, Distributor shall immediately assign or cause to be assigned (in the case of Distributor’s Affiliates) at no cost to Can-Fite, all such right, title and interest, together with any related goodwill or reputation, to Can-Fite. Distributor or its Affiliates shall promptly execute all documents reasonably requested by Can-Fite in connection with such assignment and shall use commercially reasonable efforts to cause its SubDistributors to do the same.
| - 31 - |
(f) Distributor Goodwill. The Parties agree that all use of the Trademarks by Distributor and its Affiliates and SubDistributors shall be for the sole and exclusive benefit of Distributor, and the goodwill and reputation accrued in connection with Distributor’s or any Affiliate’s or SubDistributor’s use of the Trademarks shall accrue solely to Distributor. The Parties agree that Can-Fite and its Affiliates will have no rights to use (or to authorize the use by any Third Party) any of the Trademarks. Notwithstanding the foregoing, in the event Can-Fite, or any of its Affiliates acquires any right, title or interest in or to or relating to the Trademarks for any reason, Can-Fite shall immediately assign, or cause to be assigned (in the case of Can-Fite’s Affiliates), at no cost to Distributor, all such right, title and interest, together with any related goodwill or reputation, to Distributor. Can-Fite or its Affiliates shall promptly execute all documents reasonably requested by Distributor in connection with such assignment.
(g) Infringement. Distributor agrees that only Can-Fite has the right to enjoin any infringement or registration by a Third Party of the Can-Fite Trademarks, provided, however, that Can-Fite may authorize Distributor to enjoin such infringement or registration. Distributor will assist in the prosecution of any such action as reasonably requested by Can-Fite at Can-Fite’s cost. Notwithstanding the foregoing, Can-Fite at it sole cost and expense will diligently enforce the Can-Fite Trademarks in the Territory for the benefit of Distributor.
(h) No Confusion. Distributor shall not adopt, use, or register any acronym, trademark, trade names, service mark or other marketing name that is confusingly similar to or dilutive of the Can-Fite Trademarks or the Can-Fite name, and shall not use the Can-Fite Trademarks or the Can-Fite name other than in connection with the Marketing of the Product pursuant to this Agreement. Can-Fite shall not adopt, use, or register any acronym, trademark, trade names, service mark or other marketing name that is confusingly similar to or dilutive of the Trademarks or the Distributor name, and shall not use the Trademarks or the Distributor name other than in connection with the Manufacturing and Testing of the Product pursuant to this Agreement.
8. CONFIDENTIALITY
8.1 Can-Fite’s Information.
Except as provided in Section 8.3 or elsewhere in this Agreement, Distributor shall maintain all of Can-Fite’s Confidential Information provided by Can-Fite to Distributor, whether in writing, electronically, orally or through access to Can-Fite’s premises, in strict confidence. Such information shall remain the property of Can-Fite, and Distributor shall not use the same for or on behalf of any Person or entity other than Can-Fite or make use of any such information except as permitted by this Agreement without the express prior written approval of Can-Fite.
8.2 Distributor’s Information.
Except as provided in Section 8.3 or elsewhere in this Agreement, Can-Fite shall maintain all of Distributor's Confidential Information provided by Distributor to Can-Fite, whether in writing, electronically, orally or through access to Distributor’s premises, in strict confidence. Such information shall remain the property of Distributor, and Can-Fite shall not make use of any such information except as permitted by this Agreement without the express prior written approval of Distributor.
| - 32 - |
8.3 Exceptions.
The covenants of the receiving Party contained in Section 8.1 and Section 8.2 shall not apply to Confidential Information (a) that the receiving Party can reasonably demonstrate by competent proof is required to be disclosed by Law or a court or other Authority pursuant to (i) regulatory filings; (ii) prosecuting or defending litigation; (iii) complying with applicable Law and orders or decisions of any Official Body having jurisdiction; (iv) necessary to the limited extent only to conducting pre-clinical or clinical trials of Product and persons involved in such trials are bound by similar terms of confidentiality; or (b) disclosed to directors, officers, employees, representatives, or Affiliates who agree to be bound by similar terms of confidentiality. Notwithstanding any provision herein to the contrary, nothing herein shall prevent or prohibit any disclosure of any information concerning this Agreement (A) required under applicable securities Laws and the rules and regulations of any stock exchange or market system on which any Party’s securities are or may be traded, (B) by either Party in connection with an Approved Transaction (as defined below), where prospective parties or the other party or parties to such Approved Transaction have entered into confidentiality agreements with the Party concerning such Confidential Information, (C) to either Party’s financial advisors or legal advisors who have agreed to the limitations on disclosure contained herein and/or (D) to investment bankers and/or financing sources in connection with bona fide financing transactions involving either Party or an Affiliate. For the purposes of this Agreement, each of the following shall constitute an “Approved Transaction”: (i) the issuance by either Party of securities in connection with any financing transaction or public offering, and/or (ii) a merger, consolidation or other similar transaction involving either Party (i.e., wherein another entity acquires all or substantially all of that Party’s equity interests or assets or a merger or consolidation or similar transaction wherein securities of the post transaction entity will be issued to the other party). If a Party is required or permitted to make a disclosure of the other Party’s Confidential Information pursuant to this Section 8.3, it will use commercially reasonable efforts to (I) limit the scope of the Confidential Information disclosed and the number of persons to whom such Confidential Information is disclosed, in each case to the minimum extent required to address the reason such disclosure is permitted hereunder and (II) secure confidential treatment of such Confidential Information and comply with any applicable provisions of Section 12.7.
8.4 Publications.
A Party primarily responsible for a proposed publication (excluding any publication required under applicable securities Laws, which shall be subject to the provisions of Sections 8.3 and 12.7) relating to the Product in the Territory (the primary purpose of which is not advertising or promotion), whether written or oral, shall, at least thirty (30) days before presentation or submission of the proposed publication to a Third Party, submit such proposed publication to the other Party for review in connection with obtaining or preserving Intellectual Property rights and/or to determine whether such other Party’s Confidential Information is contained therein and should be modified or deleted. The receiving Party shall have thirty (30) days in which to review and consent to each proposed publication, such consent not to be unreasonably withheld or delayed. The Parties may mutually agree to extend the review period for an additional thirty (30) days when the receiving Party provides a reasonable need for such extension, including, but not limited to the preparation and filing of pertinent patent applications.
| - 33 - |
8.5 Survival.
This Article 8 shall survive termination of this Agreement for a period of three (3) years.
9. TERM AND TERMINATION OF AGREEMENT
9.1 Term.
The term of this Agreement shall commence on the Effective Date and continue for fifteen (15) years from the date of the First Commercial Sale (the “Initial Term”). This Agreement will be automatically renewed for 5 (five) year periods (each a “Renewal Term” and together with the Initial Term, the “Term”) unless either Party gives to the other Party notice of termination at least six (6) months prior to the expiry of the then current Term.
9.2 Termination.
(a) Material Breach. Except as expressly specified in this Section 9.2, this Agreement may not be terminated for a breach or otherwise, provided however, that a Party may seek to recover damages for a breach, whether or not cured, and a Party may seek specific performance for any breach of this Agreement.
(b) Distributor’s Termination Rights. Distributor may terminate this Agreement:
(i) at any time following receipt of the Regulatory Approval on ninety (90) days' prior written notice to Can-Fite;
(ii) if Can-Fite (through itself or its Affiliates) breaches its obligations under Section 2.3 such termination shall be effective upon thirty (30) days prior written notice of termination sent by Distributor to Can-Fite, provided however, that Can-Fite shall have thirty (30) days from the date of such notice to cure such breach; and such cure may be effected by cessation of the activities causing the breach and termination of any licenses or rights granted to Third Parties in breach of Section 2.3;
(iii) if a Supply Interruption occurs and Can-Fite is not able to resume supply as set forth in Section 6.3(c).
| - 34 - |
(c) Can-Fite’s Termination Rights. Can-Fite may terminate this Agreement:
(i) If Distributor fails to pay any amounts due to Can-Fite under this Agreement in excess of $20,000 (due and owing at one time) when due; provided however, such amounts are not subject to setoff as provided in Section 12.2; and provided further, such payments are not subject to a bona fide dispute; provided, further, that if Distributor pays the amounts due and payable pursuant to Section 6.7(a)(iii) or (iv), in each case, in accordance with Distributor’s good faith calculations, and Can-Fite disagrees with those calculations and/or requests an audit with respect to such calculations, any amounts in excess of such good faith calculations shall be payable pursuant to clause (B) of the following sentence. Such termination shall be effective, (A) in the case of (I) failure to make payments not subject to a bona dispute or (II) failure of Distributor to make payments under Sections 6.7(a), in each case, to the extent of Distributor’s good faith calculations, upon ten (10) days prior written notice of termination sent by Can-Fite to Distributor, provided that Distributor shall have (10) ten days from receipt of notice of termination to cure such default, and (B) in the case of all other payment defaults ten (10) days following Distributor’s receipt of written notice of termination provided by Can-Fite sent after resolution of the payment dispute (by agreement between the Parties, through the mechanism of Section 6.8, or through the mechanism of Section 12.2, as applicable). provided however that Distributor shall have ten days from receipt of such notice of termination to cure such default;
(ii) if Distributor or its Affiliates breaches its obligations under Sections 2.2, 2.3 or 8, such termination shall be effective upon thirty (30) days prior written notice of termination sent by Can-Fite to Distributor, provided however, that Distributor shall have thirty (30) days from the date of such notice to cure such breach; and such cure may be effected by the immediate cessation of the activities causing the breach and termination of any licenses or rights granted to Third Party in breach of such Section 2.2 or 2.3;
(iii) if First Commercial Sale is not achieved within one year from the NDS; provided, however, that Can-Fite shall not be entitled to terminate this Agreement if failure to achieve such First Commercial Sale results from a failure of Can-Fite to supply Product or another circumstance beyond the control of Distributor, including, without limitation, a Force Majeure or a change in applicable Law; or
(iv) in the event the minimum sales requirements set forth in Schedule D are not achieved.
(d) Bankruptcy and Insolvency. A Party shall have the right to terminate this Agreement in the event that a court of competent jurisdiction declares the other Party insolvent or bankrupt, or a bankruptcy proceeding is commenced against the other Party that is not dismissed within ninety (90) days of commencement or the other Party files a proposal, assignment for the benefit of creditors, arrangement, composition or seeks similar relief under any applicable bankruptcy law or the other Party is in receivership, in which case termination shall be effective upon written notice to that effect.
| - 35 - |
9.3 Accrued Rights, Surviving Obligations.
Termination or expiration of this Agreement shall not affect any accrued rights of either Party or payments otherwise owing. Without limiting the foregoing, the terms of Sections 4.8, 5.1, 5.2, 5.3, 5.4, 6.7 (to the extent of amounts owed that accrued during the Term and amounts owed for sale of unsold Product (percentage of Net Sales) and unsold Authorized Generic (percentage of Net Profits) under Section 9.4), 6.8 and 6.9 (only with respect to specific issues relating to quality of Supplied Product delivered to Distributor during the Term) and Articles 7 (excluding Section 7.7 with respect to actions commenced post-termination, but including such section for actions commenced pre-termination (but only to the extent of pre-termination infringement, with any damages for post-termination infringement, accruing only to Can-Fite (as opposed to Distributor) and with Distributor having no obligation to pursue such post-termination damages) and except that the provisions of Section 7.4 shall survive only to the extent a claim, suit or action arises out of Distributor’s exercise of rights under this Agreement), 8, 9, 10, 11, and 12 of this Agreement shall survive termination or expiration of this Agreement.
9.4 Transitional Matters.
(a) Upon expiration or termination of the Agreement, Distributor may, where permitted by Law, sell Supplied Product then in its inventory for a period of [...] months thereafter, all in accordance with the terms of this Agreement. [...]. Can-Fite will have the right to cancel any purchase orders placed by Distributor which were accepted by Can-Fite prior to such termination, and which require delivery of Supplied Product after the date of termination including during such twelve (12) month period.
(b) Upon termination, Distributor and Can-Fite shall at their own expense make Commercially Reasonable Efforts to ensure that the continuity of patient care is not disrupted including transferring of managed care contracts, adverse event reporting, and dealing with supply chain matters. In addition, Distributor will remain responsible for returned Supplied Product sold by Distributor and Can-Fite will be responsible for returned Supplied Product following termination of this Agreement provided it was not sold by Distributor pursuant to this Agreement, including Section 9.4(a) above. For the purpose of identifying the responsible party, Supplied Product will be tracked via lot numbers.
9.5 Transfer of Approvals.
Within thirty (30) days of expiration or termination of the Agreement, Distributor shall transfer or cause to be transferred to Can-Fite, or to any Third Party designated by Can-Fite, all Approvals relating to Supplied Product, including all Regulatory Approvals, the New Drug Submission(s), Other Approvals, and Can-Fite Trademarks and Trademarks specifically relating to the Product or Authorized Generic, or applications therefore, that are in the name of Distributor, at Can-Fite’s cost.
| - 36 - |
9.6 Effect of Termination.
Termination of this Agreement shall not operate to release either Party from any obligation or liability incurred under this Agreement prior to or upon termination hereof or which, by the terms hereof, survives termination. Upon any termination of this Agreement, except to the extent required for the purposes of Section 9.4, (i) all licenses and rights granted to Distributor hereunder shall immediately terminate and (ii) all rights, properties and interests granted by Can-Fite to Distributor shall immediately revert to and become fully vested in Can-Fite and Distributor shall return to Can-Fite all copies of documents regarding Supplied Product and all Confidential Information supplied by Can-Fite. For the avoidance of doubt, the Parties acknowledge that Can-Fite shall have no rights to the Trademarks following any termination of this Agreement.
9.7 License Survival During Bankruptcy.
The Parties agree that Distributor, as a licensee of such rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the Israeli bankruptcy law subject to performance by Distributor of its obligations under this Agreement. The parties further agree that, in the event Can-Fite elects to reject or terminate this Agreement while Can-Fite is the subject of a case or proceeding for bankruptcy and Distributor elects to continue the licenses under this Agreement as contemplated by the preceding sentence, then Distributor shall be entitled, upon reasonable request, to have access, in confidence, to such of Product Know How not already in Distributor’s possession, as shall be reasonably necessary to make use of the license rights under this Agreement without participation by Can-Fite, subject to the terms and conditions of this Agreement, including without limitation, Sections 2.2, 2.3 and 2.4. The licensor of intellectual property to Distributor under this Agreement shall be (i) an Israeli entity or (ii) an entity of a jurisdiction having legal protections in the event of a bankruptcy or reorganization filing of the licensor, which in the reasonable opinion of Distributor, are comparable or at least as favorable to a licensee as those provided under Section 365(n) of the U.S. Bankruptcy Code, as in effect as of the Effective Date; provided, however, if the licensor is not an entity of the type contemplated in clauses (i) and (ii) above, then licensor shall place its intellectual property rights which relate to this Agreement in a trust or other structure which will provide protection to the licensee in the event of the bankruptcy or reorganization filing of the licensor at a level at least comparable to that contemplated in clauses (i) or (ii) above; and provided, further, that such trust or similar structure must be reasonably acceptable to Can-Fite and Distributor.
| - 37 - |
10. INDEMNITY
10.1 Indemnification by Can-Fite.
Can-Fite agrees to and hereby does indemnify, defend and hold the Distributor Indemnitees harmless from and against all losses, claims, damages, costs and expenses, including reasonable attorneys’ fees (including, without limitation, those resulting from Third Party claims, actions, or proceedings) (collectively “Losses”) arising from: (a) the breach of any representation, warranty, covenant or obligation by Can-Fite hereunder (except for that relating to Third Party infringement indemnification, the provisions for which are exclusively limited to Sections 7.4 and 7.5), (b) any negligent act or omission, or willful misconduct of Can-Fite or its Affiliates or any of its Approved Manufacturers or, as applicable, any of its Contract Finishers; (c) the Marketing of Supplied Product inside or outside the Territory by Can-Fite or any of its Affiliates; (d) the failure of the Supplied Product sold to Distributor to meet the Specifications; or (e) any Product Liability Claims, not covered by subsections (a)-(d) above or subsections (a)-(g) of Section 10.2 (to the extent of fifty percent (50%) of such Losses); provided, however, that in the case of each of subsections 10.1(a), (c), (d), or (e) Can-Fite shall have no indemnity obligation to Distributor for any Losses for which Distributor is obligated to indemnify Can-Fite pursuant to Section 10.2 if the Losses are primarily and substantially a result of the facts giving rise to Distributor’s obligation to indemnify.
10.2 Indemnification by Distributor.
Distributor agrees to and hereby does indemnify and hold the Can-Fite Indemnitees harmless from and against all Losses (unless other specified) arising from: (a) the Marketing, Testing, sale or other distribution of Supplied Product by Distributor or its SubDistributors or any of their respective Affiliates or agents in violation of the terms of this Agreement; (b) breach of any representation, warranty, covenant or obligation by Distributor hereunder; (c) any negligent act or omission, or willful misconduct of Distributor or its SubDistributors or any of their respective Affiliates or agents (d) sale or use of a pharmaceutical product which is not supplied by or on behalf of Can-Fite or any of its Approved Manufacturers or any of their respective Affiliates or agents pursuant to this Agreement and which is sold or combined by Distributor or any SubDistributor with Supplied Product, (e) improper handling, storage or transport of Supplied Product by Distributor, its Affiliates, or any SubDistributor or their respective agents, (f) the unauthorized alteration, modification, or adulteration of Supplied Product by Distributor, its Affiliate, or any SubDistributor, (g) any representations or warranties made by Distributor or any of its Affiliates or SubDistributors with respect to Supplied Product (other than the labeling approved by the Regulatory Authority), or (h) any Product Liability Claims, not covered by subsections (a)-(d) of Section 10.1 or subsections (a)-(g) of this Section 10.2 (to the extent of fifty percent (50%) of such Losses), provided, however, that in the case of each of subsections 10.2(a)-(c) and (e)-(g), Distributor shall have no indemnity obligation to Can-Fite for any Loss for which Can-Fite is obligated to indemnify Distributor pursuant to Section 10.1 if the Losses are primarily and substantially a result of the facts giving rise to Can-Fite’s obligation to indemnify.
| - 38 - |
10.3 Procedure.
This Section 10.3 describes the procedure for indemnification of Losses for Third Party claims. With respect to Losses relating to the claim of a Party hereto, the procedures provided in Section 12.2 shall govern. The Party seeking indemnification for Third Party claims under Sections 10.1 or 10.2 (the “Indemnified Party”) shall promptly notify the other Party (the “Indemnifying Party”) in writing of all matters which may give rise to the right to indemnification hereunder; provided, however, that failure to promptly give the notice provided in this Section 10.3 shall not be a defense to the liability of the Indemnifying Party for such claim, but the Indemnifying Party may recover any actual Losses arising from the Indemnified Party’s failure to give such prompt notice. The Indemnified Party shall not admit any liability with respect to, or settle, compromise or discharge any such matter covered by this Article 10 without the Indemnifying Party’s prior written consent (which shall not be unreasonably withheld, delayed or conditioned). The Indemnifying Party shall have the right, with the consent of the Indemnified Party (which shall not be unreasonably withheld, delayed or conditioned), to settle all indemnifiable matters under this Article 10 related to claims by Third Parties which are susceptible to being settled. In connection with any claim giving rise to indemnity under this Article 10 resulting from or arising out of any claim or legal proceeding by a Person other than the Indemnified Party, the Indemnifying Party at its sole cost and expense may, upon written notice to the Indemnified Party and an acknowledgement of its indemnity obligations hereunder, assume the defense of any such claim or legal proceeding. If the Indemnifying Party assumes the defense of any such claim or legal proceeding, the Indemnifying Party shall select counsel reasonably acceptable to the Indemnified Party to conduct the defense of such claims or legal proceedings and, at the Indemnifying Party’s sole cost and expense (which costs and expenses shall not be applied against any indemnity limitation herein), shall take all steps necessary in the defense or settlement thereof. The Indemnified Party shall be entitled to participate in (but not control) the defense of any such action, with its own counsel and at its own expense, and shall be entitled to any and all information and documentation relating thereto. If the Indemnifying Party does not assume (or continue to diligently and competently prosecute) the defense of any such claim or litigation resulting therefrom in accordance with the terms hereof, the Indemnified Party may, at the Indemnifying Party’s expense, defend against such claim or litigation in such manner as it may deem appropriate, but may not settle such claim or litigation without the consent of the Indemnifying Party, which consent shall not be unreasonably withheld, delayed or conditioned. The Indemnified Party will cooperate reasonably with the Indemnifying Party in its efforts to conduct or resolve such matters, including by making available to the Indemnifying Party relevant documents and witnesses. The Indemnified Party and the Indemnifying Party shall keep each other informed of all settlement negotiations with Third Parties and of the progress of any litigation with Third Parties. The Indemnified Party and the Indemnifying Party shall permit each other reasonable access to books and records and shall otherwise cooperate with all reasonable requests of each other in connection with any indemnifiable matter resulting from a claim by a Third Party.
10.4 Indemnification Not Sole Remedy.
Each Party hereby acknowledges that the indemnification provided under this Article 10 shall in no manner limit, restrict or prohibit (unless liability is otherwise expressly limited by the terms of this Agreement) either Party from seeking any recovery or remedy provided at law or in equity from the other Party in connection with any breach or default by such other Party of any representation, warranty or covenant hereunder, including injunctive relief.
| - 39 - |
10.5 Insurance.
Each Party will have in force prior to the First Commercial Sale and shall maintain in good standing throughout the Term of this Agreement and for a period of seven (7) years thereafter, product liability insurance policies in respect of the Supplied Product(s) with an internationally recognized insurer or insurers licensed to do business in the Territory in an amount not less than $5 million per occurrence, on such terms and conditions as are customary in the industry, and shall list the other Party as an additional insured on such policy(ies). Each Party shall provide proof of such insurance to the other Party within ten (10) days prior to the First Commercial Sale and thereafter from time to time within thirty (30) days of request of proof of such insurance.
11. REPRESENTATIONS, WARRANTIES AND COVENANTS; LIMITATIONS OF LIABILITY
11.1 Representations, Warranties and Covenants.
(a) Organization and Authority. Each Party represents and warrants that it (i) is duly organized, validly existing and, in the case of Cipher only, in good standing under the Laws of the jurisdiction of its organization, (ii) is qualified to do business in each other’s jurisdiction in which the conduct of its business requires such qualification, (iii) is in compliance with all applicable Laws, relating to its business and assets, and (iv) is not in material default of its memorandum or articles of association, its certificate of incorporation or by-laws or all other constituent documents as the case may be, except in the case of (ii) and (iii) where such failure to qualify or be in compliance would not have a material adverse effect on the business and assets of such Party or the performance of this Agreement by such Party.
(b) Due Authorization and Enforceability. Each Party represents and warrants that (i) it has full authority to execute, deliver and perform its obligations under this Agreement, (ii) that this Agreement has been duly executed and delivered by such Party, and constitutes the legal, valid and binding obligations of such Party and is enforceable against such Party in accordance with its terms, and (iii) that the execution, delivery and performance of this Agreement will not violate, be inconsistent with or result in a default under or creation of lien or encumbrance under (except as specifically contemplated by this Agreement) (A) the memorandum or articles of association, certificate of incorporation or by-laws or other constituent documents, as the case may be, of any Party and/or its Affiliates, (B) any material agreement, contract, license understanding or instrument binding upon or affecting such Party or its properties or assets, whether express, implied, written or oral, or (C) any applicable Laws affecting either Party or its properties or assets, except where such violation would not have a material adverse effect on the business and assets of such Party.
(c) Import and Product Handling. Each Party covenants that it will and will cause its Affiliates and agents and, in the case of Distributor, its SubDistributors and, in the case of Can-Fite, its Approved Manufacturer(s) and, if applicable, Contract Finisher(s) to, comply with all applicable Laws relating to the importation, warehousing, storage, Manufacturing, Marketing, Packaging and Testing of Supplied Product applicable to such Laws and will ensure that all required Approvals are in effect and will maintain such Approvals in good standing.
(d) Rights to Grant. Can-Fite represents and warrants that it has the sole, exclusive and unencumbered right to grant the rights (including any license) herein granted to Distributor, and that neither Can-Fite, nor any other Person, has granted any option, license, right or interest in or to the Product to any Third Party which could conflict with the rights granted by it under this Agreement in the Territory.
| - 40 - |
(e) Trademarks. Can-Fite represents and warrants that, to its knowledge, it has all rights to the Can-Fite Trademarks and that such trademarks are valid. Distributor represents and warrants that, to its knowledge, it has all rights to the Trademarks and such trademarks are valid.
(f) Intellectual Property. Can-Fite represents, warrants and covenants that it owns or has the exclusive license to all intellectual property (including all Intellectual Property), assets, licenses and approvals necessary to make, have made, use, sell, offer for sale and import the Products for use in the Field on an exclusive basis and will possess all such rights during the Term as may be necessary to promote, market, distribute and sell the Product for use in the Field in the Territory itself and/or through Distributor or its Affiliates as contemplated by this Agreement. Can-Fite further represents, warrants and covenants that it has the right and ability to initiate patent litigation against Third Parties based upon an infringement of the Product Patents. Can-Fite represents and warrants that there is no other intellectual property (including, without limitation, any license to same) required for Can-Fite to Market, Manufacture or have Manufactured, Package or have Packaged, or Test or have Tested Product, or to grant Distributor the rights provided in this Agreement.
(g) No Claims. Can-Fite represents, warrants and covenants that there are no proceedings currently pending or, to the knowledge of Can-Fite, threatened against, Can-Fite or any of its Affiliates, relating to or otherwise arising from (i) Product Liability Claims or claims for death or bodily injury relating to any Product, or (ii) infringement, misappropriation or other conflict with any intellectual property or other rights of any Person relating to any Product or any Can-Fite Trademarks, or (iii) the promotion, marketing, manufacture, distribution or sale of any Products.
(h) No Infringement. Can-Fite represents and warrants that (i) to its knowledge, the Product does not and will not infringe the intellectual property rights of any Person; (ii) to its knowledge, Distributor’s Marketing, sale and distribution of the Product as contemplated by this Agreement shall not infringe or otherwise violate the intellectual property rights of any person; and (iii) to its knowledge, the Manufacture and packaging of Product by an Approved Supplier including Distributor will not infringe the intellectual property rights of any Person.
11.2 Quality Assurance Representations, Warranties and Covenants.
(a) Can-Fite, in its own name and on behalf of any of its Approved Manufacturers, Contract Finishers and Affiliates engaged in the performance of the actions contemplated hereby, including the Manufacture, sale and delivery of Supplied Product hereunder, hereby represents, warrants and covenants to Distributor that all Supplied Product that Can-Fite or any of Can-Fite’s Approved Manufacturers or Affiliates Manufactures, supplies and delivers under and pursuant to this Agreement will:
(i) conform to the Specifications at time of shipment to Distributor;
| - 41 - |
(ii) be free and clear from all liens, encumbrances and defects of title, other than those that arise directly as a result of actions taken by Distributor; and
(iii) comply in all material respects with the requirements under the GMP standards, the Regulatory Approvals and the Other Approvals, as applicable, the Act and any other applicable Law in the Territory, and will not, at the time of such delivery be adulterated or misbranded within the meaning of the Act.
(b) Can-Fite shall furnish to Distributor a certificate of analysis for each lot of the Supplied Product shipped to Distributor, and a certificate that such lot meets the quality control standards set forth in the relevant approved application for Regulatory Approval all well as the annual certificate of compliance.
(c) Distributor shall be responsible for storing Supplied Product under appropriate conditions as specified in labeling and for distribution in full compliance with the applicable GMP standards, the Regulatory Approvals, the Other Approvals, the Act and the applicable Law. Distributor shall have received and shall be in current compliance with all Approvals of any Authority as may be required to Market Supplied Product pursuant to this Agreement.
(d) None of Distributor or Distributor’s Affiliates or SubDistributors shall, in bad faith, disrupt or cause the disruption of the supply of Supplied Product into the marketplace in the Territory.
(e) Can-Fite or its Approved Manufacturer(s) shall have received, and shall at all times during the Term, be in current compliance with, all Approvals of any Regulatory Authority as may be required to Manufacture and/or to supply the Supplied Product pursuant to this Agreement, and, as of the Effective Date, Can-Fite or, to its knowledge, any Approved Manufacturer has not received any warning letters or similar regulatory letters from any Regulatory Authority within the last three (3) years with respect to the Product which Can-Fite has not disclosed to Distributor or which prevents the Manufacture and supply of the Product.
(f) Can-Fite shall ensure that each lot of the Supplied Product shipped to Distributor has a shelf-life and expiration date of at least thirty-six (36) months at the date of shipment.
(g) Each Party represents and warrants to the other Party that it has not engaged in any conduct or activity which could justify an FDA debarment action, and no debarment proceedings are currently underway or, to its knowledge, contemplated against it or any of its employees and, to its knowledge, neither its Approved Manufacturer or any of its Contract Finishers has engaged in conduct that would justify an FDA debarment action and no proceedings are currently underway or contemplated against any of its Approved Manufacturers or Contract Finishers or any of their employees.
11.3 Distributor’s Compliance with Laws.
Without limiting anything herein, Distributor shall comply with all applicable Laws in performing this Agreement, including all Marketing, promotional or advertising activities conducted by its Afiliates, or SubDistributors or its or their agents.
| - 42 - |
11.4 Limitation of Liability.
EXCEPT AS EXPRESSLY SET FORTH IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATION OR WARRANTY TO THE OTHER PARTY OF ANY KIND, INCLUDING WITHOUT LIMITATION ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE.
NOTWITHSTANDING ANYTHING TO THE CONTRARY CONTAINED IN THIS AGREEMENT, EXCEPT WITH RESPECT TO CONFIDENTIALITY AND INDEMNIFICATION OBLIGATIONS AS SET FORTH IN THIS AGREEMENT, IN NO EVENT WILL EITHER PARTY BE LIABLE TO THE OTHER FOR ANY INCIDENTAL, CONSEQUENTIAL, SPECIAL, PUNITIVE, INDIRECT DAMAGES, LOSS OF PROFIT, LOSS OF REVENUE, LOSS OF USE EVEN IF INFORMED OF POSSIBILITIES OF SUCH DAMAGES OR LOSSES.
12. MISCELLANEOUS
12.1 Governing Law.
This Agreement shall be governed by laws of New York, excluding its choice of law provisions. The Parties hereby agree to exclude the application of the International Sale of Goods Act.
12.2 Dispute Resolution.
The Parties recognize that any dispute, controversy or claim arising under or relating to this Agreement, (collectively, a “Dispute”) may from time to time arise during the Term of this Agreement including in relation to the selection and appointment of the Third Party Laboratory and Auditor. It is the objective of the Parties to establish procedures to facilitate the resolution of disputes arising under this Agreement in an expedient manner by mutual cooperation and without resort to litigation. To accomplish this objective, the Parties agree to follow the procedures set forth in this Section 12.2 if and when a dispute arises under this Agreement. Each Party shall designate an individual (the “Responsible Person”) to whom disputes shall be initially referred. Such Responsible Person shall be (i) in the case of Can-Fite, a person having managerial responsibility in the functional area of dispute and (ii) in the case of the Distributor, a person having managerial responsibility in the functional area of dispute. Disputes under this Agreement between the Parties shall be submitted to the other Party’s Responsible Person. The Responsible Persons will meet and hear the disputed matter in as timely a manner as possible. If the Responsible Persons are unable to reach a decision within ten (10) days, such matter shall be referred for resolution to the Chief Executive Officer of Can-Fite and the Chief Executive Officer of Distributor (or such other officer exercising the duties of such office). Such submission shall be made with each Party submitting a statement as to the disputed matter and the Responsible Persons providing a joint statement as to which matters they were unable to agree upon. In the event that a dispute is not resolved by the foregoing procedures within thirty (30) days of first being submitted by a Party, the matter shall be finally settled exclusively by arbitration under the International Arbitration Rules of the American Arbitration Association (the “Rules”), provided however that nothing herein shall prevent or prohibit any Party from seeking injunctive relief or non-monetary equitable relief as permitted in any court within appropriate jurisdiction. Either Party may, by written notice to the other, have a Dispute referred to arbitration. Unless otherwise agreed in writing, any arbitration shall be conducted in the English language and shall be held in New York City, New York, and heard by a panel of three (3) arbitrators. Each party shall nominate one arbitrator. The third arbitrator, who will be chairman of the arbitral tribunal, shall be appointed in accordance with the Rules. The decision and award of the arbitral tribunal shall be final and binding and may be entered in any court of competent jurisdiction, for which purpose, and for all other purposes under this Agreement, each party hereto submits to the exclusive jurisdiction and venue of the U.S. District Court for the Southern District of New York, or in the absence of jurisdiction of such court to the Supreme Court of New York County. The costs of any such arbitration shall be allocated as follows: (i) if the arbitrators rule in favor of one Party on all disputed issues in the arbitration, the losing Party shall pay one hundred percent (100%) of such out-of-pocket fees and expenses; and (ii) if the arbitrators rule in favor of one Party on some issues and the other Party on other issues, the arbitrators shall issue with the ruling a written determination as to how such fees and expenses shall be allocated between the Parties. At Distributor’s request (but not absent such request) (i) the arbitrators may offset any amounts owed to Distributor against amounts owed by Distributor to Can-Fite, or may reduce the royalty rate on Net Sales or share of Net Profits so that Distributor may recoup amounts through the reduced royalty rate. At Can-Fite’s request (but not absent such request) (i) the arbitrators may offset any amounts owed to Can-Fite against amounts owed by Can-Fite to Distributor or may increase the royalty rate on Net Sales or share of Net Profits so that Can-Fite may recoup amounts through the increased royalty rate. In no event may the arbitrators terminate this Agreement other than as provided in Section 9.2 or otherwise award punitive, special or consequential damages. The arbitrators shall allocate fees and expenses in a way that bears a reasonable relationship to the outcome of the arbitration, with the Party prevailing on more issues, or on issues of greater value or gravity, recovering a relatively larger share of its legal fees and expenses. Each Party is required to continue to perform its obligations under this Agreement pending final resolution of the Dispute; provided that Can-Fite is only required to continue to supply Distributor with Supplied Product and replace rejected Supplied Product if Distributor makes payment for Supplied Product except as expressly provided for in this Agreement, including Sections 6.5 and 6.6 hereof. In no event will a demand for arbitration hereunder be made after the date when institution of a legal or equitable proceeding based upon such Dispute would otherwise be barred by the applicable statute of limitations. If a Dispute relating to payments is resolved by agreement of the Parties or by arbitration, and if the amount is not paid within ten (10) days of the Dispute resolution, the party entitled to payment (the “Prevailing Party”) may setoff some or all of such amount against amounts it owes the other Party and in the case Distributor is the Prevailing Party it may in addition setoff the amount against future payments.
| - 43 - |
12.3 Entire Agreement; Amendments.
This Agreement, including the Schedules hereto, sets forth the entire terms of the supply and distribution arrangement between the Parties hereto and, except as otherwise set forth herein, supersedes and terminates all prior agreements and understandings between the Parties. No subsequent alteration, amendment, change or addition to this Agreement shall be binding upon the Parties unless reduced to writing and signed by an authorized officer of each Party.
12.4 Tax Withholding.
Can-Fite shall be liable for all income and other taxes (including interest) (“Taxes”) imposed upon any payments made by Distributor to Can-Fite under this Agreement. If applicable laws, rules or regulations require the withholding of Taxes, Distributor shall make such withholding payments and shall subtract the amount thereof from payments under this Agreement; provided that the Parties shall use all reasonable and legal efforts to minimize tax withholding on payments made to the other Party hereunder. Distributor shall submit to Can-Fite appropriate proof of payment of the withheld Taxes as well as the official receipts within a reasonable period of time not to exceed sixty (60) days following the date of the Tax payment. Distributor shall provide Can-Fite reasonable assistance, which shall include the provision of such documentation as may be required by the tax authority, in order to allow Can-Fite to obtain the benefit of any present or future treaty against double taxation which may apply to such payments or to claim an exemption from or obtain a repayment or a reduction of such tax.
12.5 Notices.
When a Party is required or permitted to give notice under this Agreement, the notice shall be in writing, shall specifically refer to this Agreement and shall be deemed to have been sufficiently given and received for all purposes within (i) two (2) days if the Party sent the notice by internationally recognized express delivery service, (ii) one (1) day if the Party sent the notice by email, with acknowledgement of receipt and a prompt follow-up copy sent by an internationally recognized express delivery service, or (iii) immediately if the Party personally delivered the notice. Unless otherwise specified in writing, the mailing addresses of the Parties shall be as set forth below.
For Can-Fite:
Can-Fite Pharmaceuticals Inc.
10 Bareket Street
Kiryat Matalon
P.O. Box 7537
Petah-Tikva 49170
Israel
Email: pnina@canfite.co.il
Attention: Pnina Fishman
With a copy (which shall not constitute notice) sent simultaneously to:
Sichenzia Ross Friedman Ference LLP
61 Broadway, 32nd Floor
New York, NY 10006
Email: gsichenzia@srff.com
Attention: Gregory Sichenzia
| - 44 - |
For Distributor:
Cipher Pharmaceuticals Inc.
Tomken Road, Unit 16
Mississauga Ontario L4W 4P1
Email: sobrien@cipherpharma.com
Attention: Shawn Patrick O’Brien
With a copy (which shall not constitute notice) sent simultaneously to:
Torys LLP
Suite 3000, P.O. Box 270 79
Wellington Street West TD Centre
Toronto, ON M5K 1N2
E-mail: creicin@torys.com
Attention: Cheryl V. Reicin
Copies (other than to outside counsel) are an integral part of notice. Notices of termination may only be sent by internationally recognized express delivery service or by personal delivery.
12.6 Assignment.
Neither Party shall assign or otherwise transfer this Agreement or any interest herein or right or obligation hereunder without the prior written consent of the other Party not to be unreasonably withheld, and any such purported assignment, transfer or attempt to assign or transfer any interest herein or right hereunder shall be void and of no effect; provided, however, that a Party may assign its rights and obligations hereunder to an Affiliate or to a transferee or acquirer of, or successor to, its assets or securities in the event of a merger, sale of stock, sale of assets or other transaction without the prior consent of the other Party; provided, further that (i) in the case of an assignment to an Affiliate, the assigning Party shall remain responsible for all of its obligations and agreements set forth herein, notwithstanding such assignment, and (ii) in the case of a merger, sale of stock, sale of assets, assignments or other transaction, such transferee or successor shall assume in writing the obligations of the party to which it is the transferee or successor, including, without limitation, those set forth in Sections 2.2, 2.3 and 2.4 hereof; and (iii) either Party may have its Affiliates perform its obligations hereunder; provided that the delegating Party shall remain responsible for all of its obligations and agreements set forth herein, notwithstanding such delegation. Notwithstanding the foregoing, the licensor of intellectual property to Distributor under this Agreement shall be an entity of a jurisdiction having legal protections in the event of a bankruptcy or reorganization filing of the licensor, which in the reasonable opinion of Distributor, are comparable or at least as favorable to a licensee as those provided under Israeli law, as in effect as of the Effective Date.
| - 45 - |
12.7 Public Announcements.
Neither Party shall make any publicity releases, interviews or other dissemination of Confidential Information concerning Supplied Product (the purpose of which is not advertisement or promotion), this Agreement or its terms, or either Party’s performance hereunder, to communication media, financial analysts or others without the prior written approval of the other Party, which approval shall not be unreasonably withheld, conditioned or delayed. Either Party may, upon written notice to the other, make any disclosure in filings with Authorities as required by Law or applicable court or other order; provided, however, that the other Party shall have not less than three (3) Business Days to review and comment on such disclosures and filings unless a shorter period is necessitated by securities laws.
12.8 Severance.
If any Official Body or Regulatory Authority having jurisdiction over either Can-Fite or Distributor declares any Article or part thereof invalid or any such Official Body or Regulatory Authority deems any Article or part thereof to be contrary to any Laws, then such Article or part thereof shall be deemed stricken from this Agreement in that jurisdiction. To the extent possible the Parties shall revise such invalidated Article or part thereof in a manner that will render such provision valid without impairing the Parties’ original intent.
12.9 Non-Waiver.
The failure of a Party in any one or more instances to insist upon strict performance of any of the terms and conditions of this Agreement shall not be construed as a waiver or relinquishment, to any extent, of the right to assert or rely upon any such terms or conditions on any future occasion. Except as otherwise specified, all rights, remedies, undertakings, obligations and agreements contained in this Agreement shall be cumulative and none of them shall be a limitation of any other remedy, right, undertaking, obligation or agreement.
12.10 Further Assurances.
Each Party hereto agrees to execute such further documents and take such further steps as the other Party reasonably determines may be necessary or desirable to effectuate the purposes of this Agreement.
12.11 Force Majeure.
No Party shall be in breach of this Agreement, or liable to the other Party, for any delay or failure of performance to the extent such delay or failure is caused by Force Majeure, provided that the Party claiming Force Majeure gives prompt written notice to the other Party of the occurrence of an event of Force Majeure and uses its commercially reasonable efforts to overcome the same. In the event of Force Majeure, the Parties agree to discuss the circumstances and effects thereof, including the effects on Distributor’s obligations under this Agreement, and appropriate mechanisms to address such circumstances and effects.
| - 46 - |
12.12 Anti-Corruption.
Can-Fite and Distributor each agrees that it shall comply with the requirements of applicable obligations imposed by the anti-bribery laws and foreign corrupt practices laws of all applicable jurisdictions in which the services contemplated hereunder are rendered, or obligations contemplated hereunder are carried out, and the laws of any other jurisdiction in which Can-Fite, Distributor and any of their personnel, agents or representatives conduct business in relation to dealing with payments to governments or related persons or officials, or a company, for the purpose of obtaining or retaining business for or with, or directing business to, any person. For greater certainty, the foregoing laws shall include, among others, the Foreign Corrupt Practices Act of the United States of America, the Lobbyists Registration Act (Canada), the Corruption of Foreign Public Officials Act (Canada), and the Criminal Code of Canada and any corresponding Israeli laws.
12.13 Disclaimer of Agency.
This Agreement shall not constitute either Party the legal representative or agent of the other Party, nor shall either Party have the right or authority to assume, create, or incur any Third Party liability or obligation of any kind, express or implied, against or in the name of or on behalf of another except as expressly set forth in this Agreement. None of the Distributor, its directors, officers, agents or employees shall be considered employees agents or legal representatives of Can-Fite for any purpose. None of Can-Fite, its directors, officers, agents or employees shall be considered employees agents or legal representatives of Distributor for any purpose.
12.14 Construction.
The language in all parts of this Agreement shall be construed, in all cases, according to its fair meaning. The Parties acknowledge that each Party and its counsel have reviewed and revised this Agreement and that any rule of construction to the effect that any ambiguities are to be resolved against the drafting Party shall not be employed in the interpretation of this Agreement. The words “hereof,” “herein,” “hereto” and “hereunder” and words of similar import, when used in this Agreement, shall refer to this Agreement as a whole and not to any particular provision of this Agreement. The terms defined in the singular shall have a comparable meaning when used in the plural, and vice versa. The terms “dollars” and “$” shall mean Canadian dollars. Whenever used herein, the words “include,” “includes” and “including” shall mean “include, without limitation,” “includes, without limitation” and “including, without limitation” respectively, whether or not the term “without limitation” appears after the words “include,” “includes” or “including”. The masculine, feminine or neuter gender and the singular or plural number shall each be deemed to include the others whenever the context so indicates.
| - 47 - |
12.15 Counterparts.
This Agreement shall become binding when any one or more counterparts hereof, individually or taken together, shall bear the signatures of each of the Parties hereto. This Agreement may be executed in any number of counterparts, including by email or facsimile, each of which shall be deemed an original as against the Party whose signature appears thereon, but all which taken together shall constitute but one and the same document.
12.16 Consents in Writing.
Any consents required hereunder from a Party must be in writing.
Signature Page Follows
| - 48 - |
IN WITNESS WHEREOF, the Parties hereto have duly executed this Agreement as of the date first written above.
| CAN-FITE BIOPHARMA LTD. | CIPHER PHARMACEUTICALS INC. | |||
| By: | /s/ Pnina Fishman | By: | /s/ Shawn O’Brien | |
| Name: | Dr. Pnina Fishman | Name: | ||
| Title: | Chief Executive Officer | Title: | ||
[SIGNATURE PAGE TO DISTRIBUTION AND SUPPLY AGREEMENT]
| - 49 - |
SCHEDULE A
CAN-FITE TRADEMARKS AND PATENTS
Trademarks
At the Effective Date Can-Fite makes use of its internal code name CF101 to designate the Product.
Can-Fite has not adopted any trademark for the Product.
Patents
At the Effective Date the Company has the following Canadian patents and patent applications relating to the Product:
Issued patent No. 2,384,111
Issued patent No. 2,434,906
Pending patent application No. 2,586,773
Issued patent No. 2,662,879
Pending patent application No. 2,761,499
Pending patent application No. 2,790,869
Pending patent application No. 2,880,753
| - 50 - |



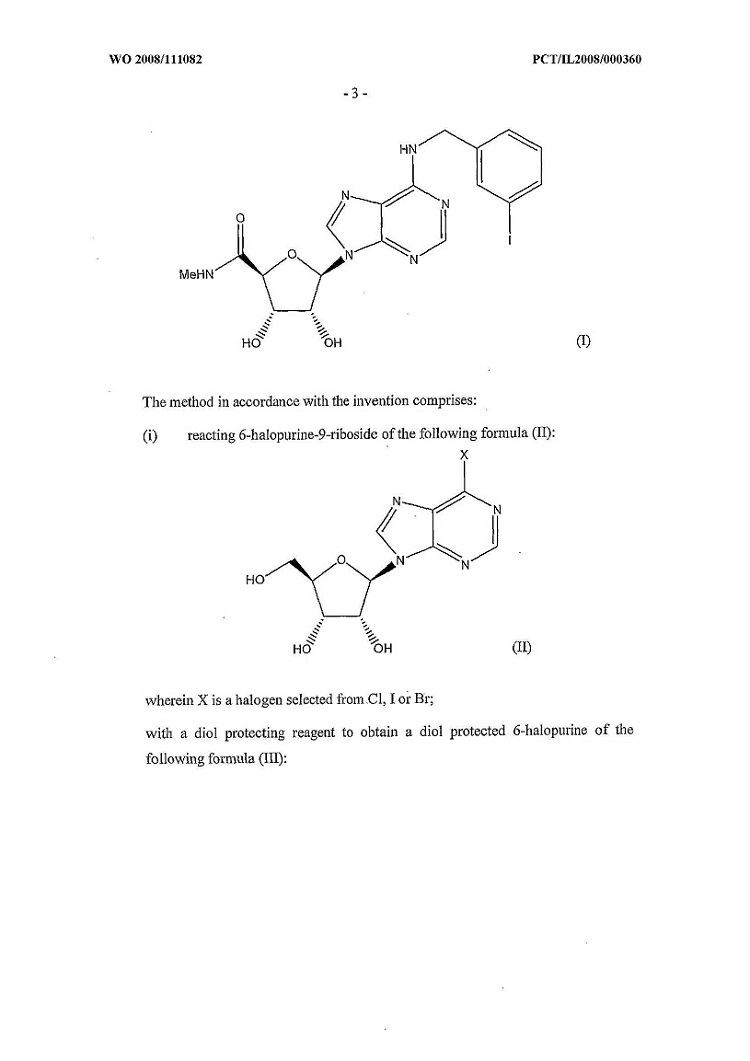
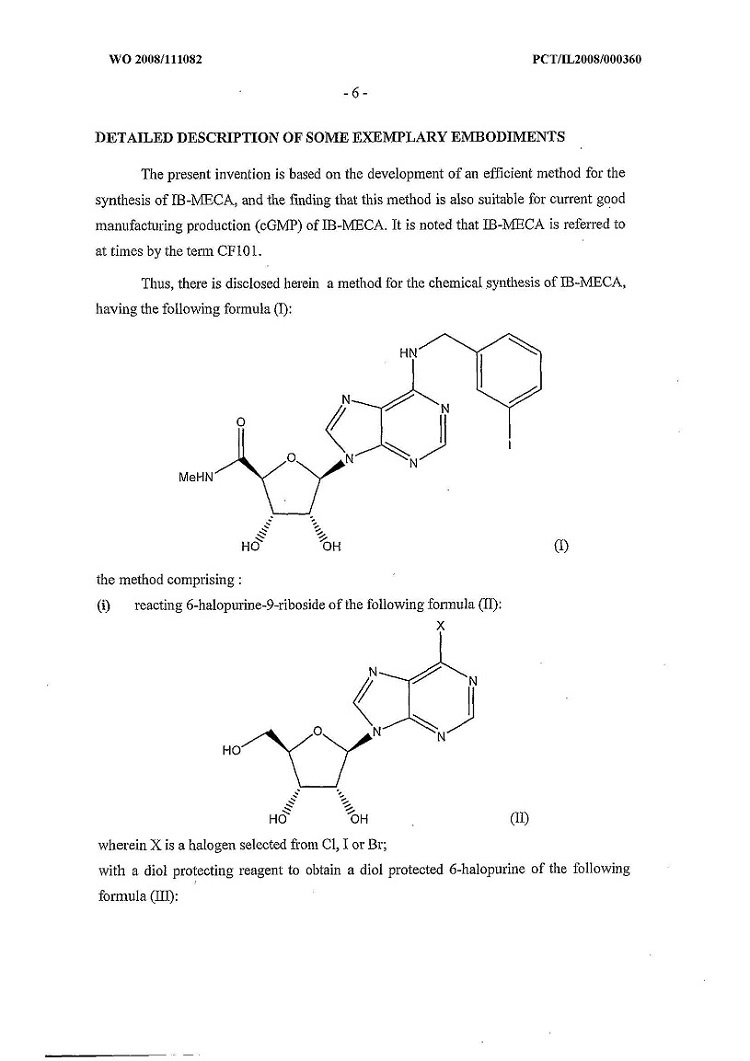












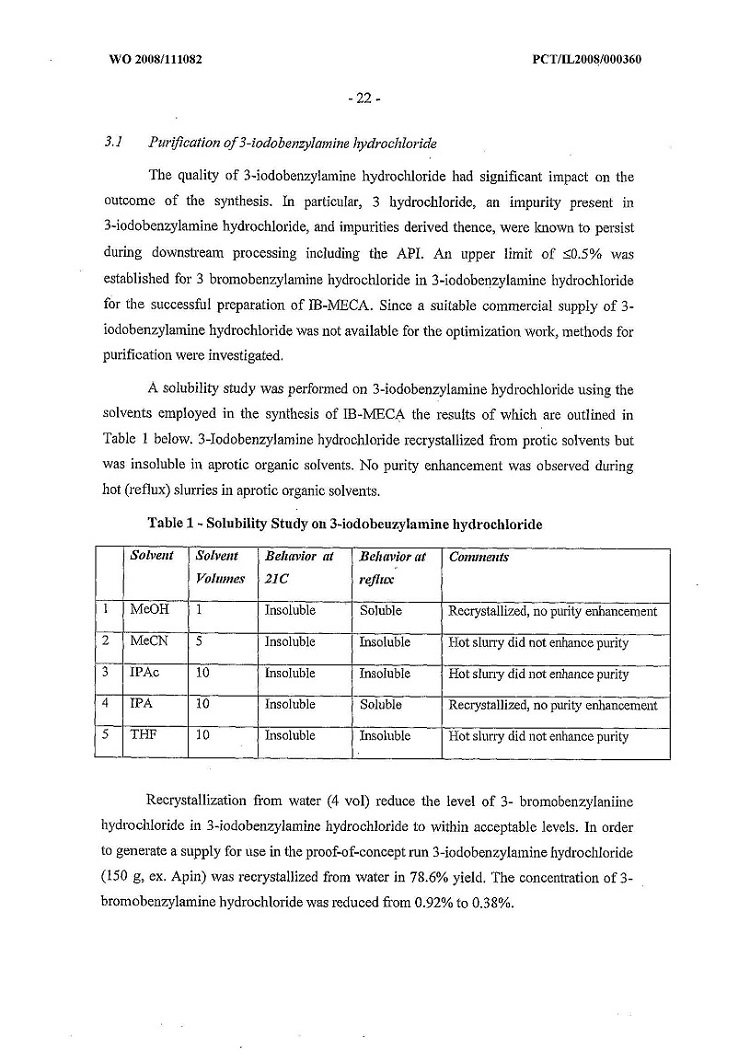













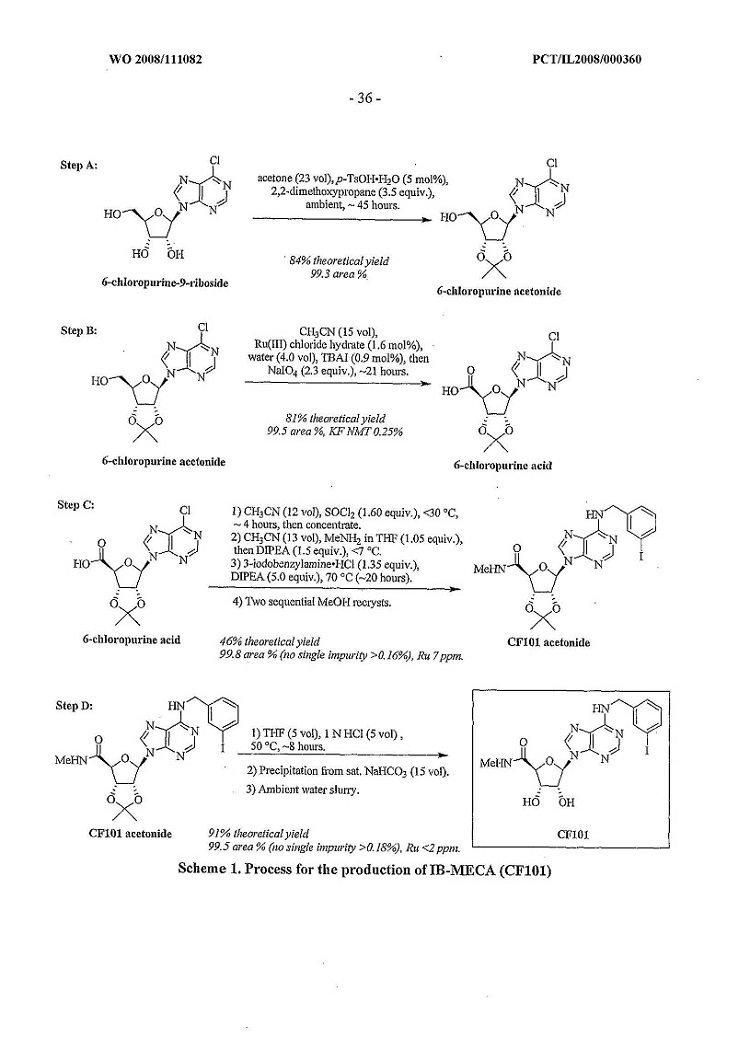
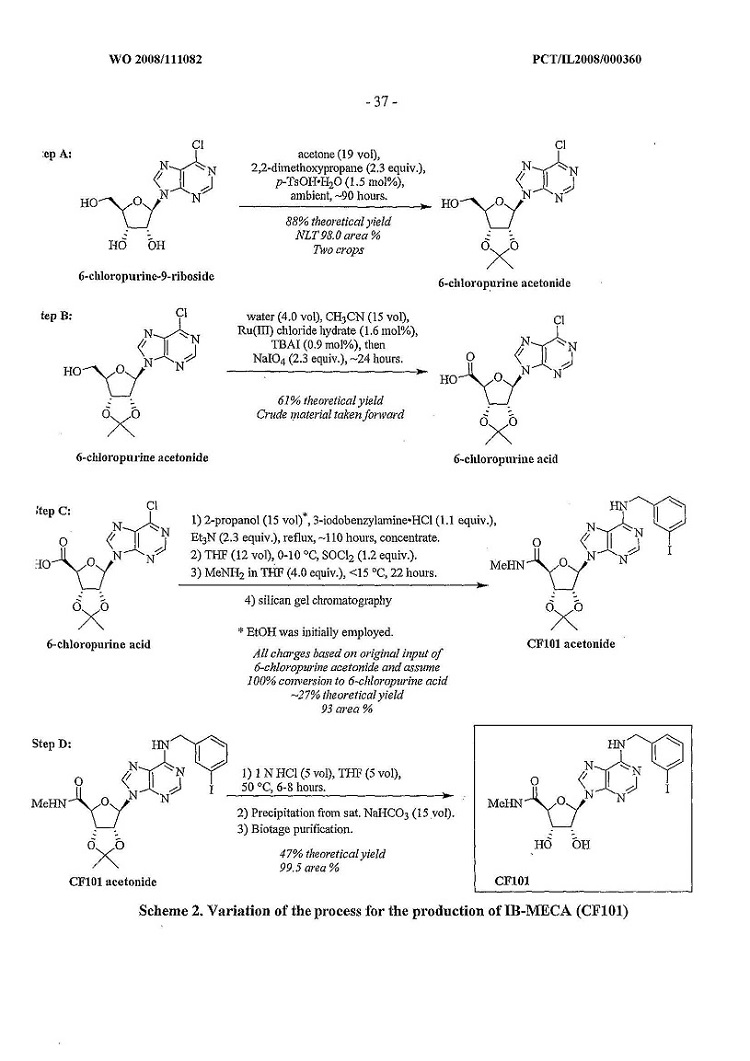


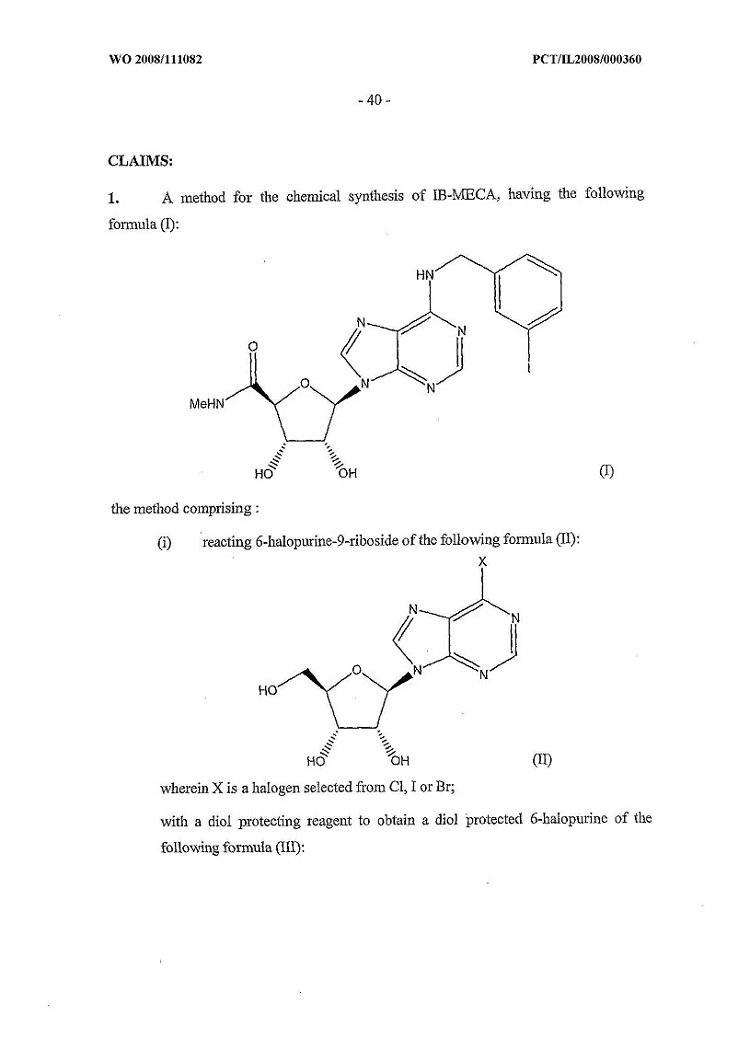






SCHEDULE D
MINIMUM SALES REQUIREMENTS
[...]