Exhibit 4. 11
Execution Copy
Confidential
LICENSE AGREEMENT
BETWEEN
CAN-FITE BIOPHARMA, LTD.
AND
SEIKAGAKU CORPORATION
DATED September 22, 2006
Execution Copy
Confidential
TABLE OF CONTENTS
| Page | ||
| ARTICLE 1. DEFINITIONS | 1 | |
| ARTICLE 2. LICENSE | 9 | |
| 2.1 | License Grant | 9 |
| 2.2 | Trademark License | 9 |
| 2.3 | Sublicenses | 10 |
| 2.4 | Right of Negotiation for Additional Exclusive License(s) within Asia | 10 |
| 2.5 | Restrictions | 10 |
| 2.6 | Retained Rights | 11 |
| 2.7 | No Implied Licenses | 11 |
| ARTICLE 3. JOINT COMMITTEE | 11 | |
| 3.1 | Joint Committee | 11 |
| 3.2 | No Committee Amendments; Authority | 12 |
| ARTICLE 4. EXCHANGE OF INFORMATION | 12 | |
| 4.1 | Information Disclosure by Can-Fite Prior to the Effective Date | 12 |
| 4.2 | Disclosure of Intellectual Property by the Parties During the Term | 13 |
| 4.3 | Information Exchange | 13 |
| 4.4 | Can-Fite’s Other Licensee(s) | 14 |
| ARTICLE 5. DEVELOPMENT; REGULATORY | 14 | |
| 5.1 | Dosage Form Development | 14 |
| 5.2 | Development Plan | 15 |
| 5.3 | Protocol of Non-Clinical Studies by Can-Fite | 15 |
| 5.4 | Development Conduct and Costs | 15 |
| 5.5 | Failure to Develop | 16 |
| 5.6 | Reference Rights; Information and Data Used for Regulatory Purposes | 17 |
| 5.7 | Manufacturing Documents | 17 |
| 5.8 | Regulatory Filings | 17 |
| 5.9 | Regulatory Communications | 18 |
| 5.10 | Product Complaints, Pharmacovigilance and Adverse Event Reporting | 18 |
| 5.11 | Compliance with Laws and Regulatory Requirements | 18 |
| 5.12 | Applications for Regulatory Exclusivity | 19 |
| 5.13 | Protocols and Regulatory Communications Obtained from Can-Fite’s Other Licensee(s) | 19 |
| ARTICLE 6. LABELING; TRADEMARKS | 19 | |
| 6.1 | Labeling | 19 |
| 6.2 | Trademarks | 19 |
| 6.3 | Display | 20 |
| 6.4 | Ownership | 20 |
| 6.5 | Termination of Use of Trademarks | 20 |
| i |
Execution Copy
Confidential
| ARTICLE 7. MANUFACTURE AND SUPPLY OF INGREDIENT | 20 | |
| 7.1 | Generally | 20 |
| 7.2 | Supply for Development Activities | 21 |
| 7.3 | Commercial Supply of the Ingredient | 21 |
| 7.4 | Transfer Price; Taxes; Shipping | 22 |
| 7.5 | Payments | 23 |
| 7.6 | Other Terms and Conditions for Supply Agreement | 24 |
| 7.7 | Option to Manufacture | 25 |
| ARTICLE 8. SALES AND MARKETING | 26 | |
| 8.1 | Marketing Efforts | 26 |
| 8.2 | Marketing Plans | 26 |
| 8.3 | Marketing Materials | 26 |
| ARTICLE 9. MILESTONES, ROYALTIES AND OTHER PAYMENTS | 26 | |
| 9.1 | Upfront and Annual Payments | 26 |
| 9.2 | Milestone Payments | 27 |
| 9.3 | Participation in Development Costs | 27 |
| 9.4 | SKK’s Data and Cost-Sharing | 29 |
| 9.5 | Royalties | 29 |
| 9.6 | Payment Method; Currency Conversion | 30 |
| 9.7 | Late Payments | 30 |
| 9.8 | Withholding Tax | 30 |
| 9.9 | Reports and Records | 31 |
| 9.10 | Records; Audit by Can-Fite | 31 |
| 9.11 | Audit by SKK | 32 |
| ARTICLE 10. INTELLECTUAL PROPERTY | 32 | |
| 10.1 | Prosecution and Maintenance | 32 |
| 10.2 | Inventions | 32 |
| 10.3 | Enforcement of Licensed Technology | 33 |
| 10.4 | Infringement of Third Party Patents | 33 |
| 10.5 | Recoveries; Settlement | 34 |
| 10.6 | Trademark Infringement | 34 |
| ARTICLE 11. REPRESENTATIONS AND WARRANTIES; LIMITATION OF LIABILITY | 35 | |
| 11.1 | Can-Fite Representations and Warranties | 35 |
| 11.2 | SKK Representations and Warranties | 35 |
| 11.3 | Disclaimer of Warranties | 35 |
| 11.4 | Limitation of Liability | 35 |
| ARTICLE 12. INDEMNIFICATION AND INSURANCE | 36 | |
| 12.1 | By Can-Fite | 36 |
| 12.2 | By SKK | 36 |
| ii |
Execution Copy
Confidential
| 12.3 | Procedure | 36 |
| 12.4 | Insurance | 37 |
| ARTICLE 13. CONFIDENTIALITY AND PUBLICITY | 37 | |
| 13.1 | Treatment of Confidential Information | 37 |
| 13.2 | Authorized Disclosure | 37 |
| 13.3 | Other Permitted Disclosures | 38 |
| 13.4 | Publicity; Terms of this Agreement | 38 |
| ARTICLE 14. TERM AND TERMINATION | 38 | |
| 14.1 | Term of this Agreement | 38 |
| 14.2 | Termination for Material Breach | 39 |
| 14.3 | Termination for Insolvency | 39 |
| 14.4 | Effect of Expiration or Termination | 39 |
| 14.5 | Return of Confidential Information | 41 |
| ARTICLE 15. DISPUTE RESOLUTION | 41 | |
| 15.1 | Negotiation | 41 |
| 15.2 | Arbitration | 41 |
| 15.3 | Court Actions; Injunctive Relief | 42 |
| ARTICLE 16. MISCELLANEOUS | 43 | |
| 16.1 | Force Majeure | 43 |
| 16.2 | Assignment | 43 |
| 16.3 | Severability | 43 |
| 16.4 | Notices | 43 |
| 16.5 | Governing Law, Venue | 44 |
| 16.6 | Entire Agreement; Amendment | 44 |
| 16.7 | Official Language | 45 |
| 16.8 | Independent Contractors | 45 |
| 16.9 | Waiver | 45 |
| 16.10 | Counterparts | 45 |
| iii |
Execution Copy
Confidential
LICENSE AGREEMENT
This License Agreement (this “Agreement”), dated as of September 22, 2006 (the “Effective Date”), is made by and between Can-Fite BioPharma, Ltd., having its principal place of business at 10 Bareket St. Petach Tikva, Israel (“Can-Fite”), and Seikagaku Corporation, having its principal place of business 6-1, Marunouchi 1-chome, Chiyoda-ku, Tokyo 100-0005, Japan (“SKK”). Can-Fite and SKK may be referred to herein individually as a “Party” and collectively as the “Parties.”
RECITALS
WHEREAS, Can-Fite is developing an adenosine A3 receptor agonist known as CF101 (as more fully described below, the “Ingredient”) for treating inflammatory diseases; and
WHEREAS, Can-Fite has initiated Can-Fite’s Phase II Clinical Trial (as defined below) of the product containing the Ingredient as the active pharmaceutical ingredient (as more fully described below, the “Product”), as described in the Existing Filing Document (as defined below); and
WHEREAS, Can-Fite owns certain intellectual property right(s) covering the therapeutic use of the Ingredient; and
WHEREAS, Can-Fite currently plans to change the dosage form of the Product from capsule to tablet; and
WHEREAS, Can-Fite desires to grant, and SKK desires to obtain, certain exclusive rights and licenses regarding the Ingredient and Product (as more specifically provided in Section 2.1 herein) within the Territory (as defined below), together with other related rights and an option to manufacture Ingredient in the Territory, all in accordance with the terms and conditions of this Agreement;
NOW THEREFORE, for and in consideration of the covenants, conditions, and undertakings hereinafter set forth, it is agreed by and between the Parties as follows:
ARTICLE 1.
DEFINITIONS
As used in this Agreement, (i) neutral pronouns and any derivations thereof shall be deemed to include the feminine and masculine and all terms used in the singular shall be deemed to include the plural and vice versa, as the context may require; (ii) the words “hereof” and “hereunder” and other words of similar import refer to this Agreement as a whole, including all exhibits, as the same may be amended from time to time, and not to any subdivision of this Agreement; (iii) the word “including” is not intended to be exclusive and means “including without limitation”; (iv) the word “days” means “calendar days,” unless otherwise stated; (iv) “Section” refers to sections and subsections in this Agreement; (iv) descriptive headings are inserted for convenience of reference only and do not constitute a part of and shall not be used in interpreting this Agreement; and the following capitalized terms shall have the following meanings:
Execution Copy
Confidential
1.1 “Affiliate” shall mean a corporation, partnership, trust, limited liability company or other entity that directly, or indirectly through one or more intermediaries, controls, is controlled by or is under common control with a Party, but only for so long as such relationship exists. For such purposes, “control” or “controlled by” and “under common control with” shall mean the possession of the power to direct or cause the direction of the management and policies of an entity, whether through the ownership of voting stock or partnership interest, by contract or otherwise. In the case of a corporation, the direct or indirect ownership of more than fifty percent (50%) of its outstanding voting shares shall in any event be deemed to confer control, it being understood that the direct or indirect ownership of a lesser percentage shall not necessarily preclude the existence of control.
1.2 “Bridging Strategy” shall mean the strategy for submission of a New Drug Application to the Regulatory Authority in Japan that involves use of results from Clinical Studies conducted outside Japan as indicated in ICH-E5.
1.3 “Can-Fite’s Other Licensee(s)” shall mean companies, firms, corporations, partnerships or other Third Party entities, to whom Can-Fite has granted a right to develop and commercialize the Product in the Field but outside the Territory.
1.4 “CDA” shall mean the Mutual Confidential Disclosure Agreement between the Parties dated as of April 27, 2004.
1.5 “Clinical Study/Studies” shall mean such clinical studies in human beings, including Phase II Clinical Trials and Phase III Clinical Trials as may be required to be conducted and/or produced by or on behalf of either Party, and (if applicable) by Sublicensee(s) or Can-Fite’s Other Licensee(s), in connection with obtaining Marketing Authorization for the Product either inside or outside of the Territory.
1.6 “Clinical Study Costs” shall mean the entire costs (including reasonable overhead) relating to the performance of a Clinical Study/Studies, including (i) payments made to contract research organizations (“CROs”), clinical trial sites, laboratories, physicians, investigators, clinical research associates (“CRAs”), consultants and other personnel directly related to the performance of a Clinical Study, (ii) costs of Ingredient and Product used in the Clinical Study, (iii) costs associated with the preparation of a final report of the Clinical Study, (iv) costs of investigator and CRA meetings relating to the Clinical Study, and (v) reasonable internal costs relating to the Clinical Study.
1.7 “Commercial Launch” shall mean the first shipping by SKK, its Affiliate, its distributor or Sublicensee of the Product following Marketing Authorization to its or their wholesalers or other Third Party purchasers in the Territory, in such commercial quantities of the Product as may reasonably be appropriate to establish the Product, as applicable, throughout the Territory.
| 2 |
Execution Copy
Confidential
1.8 “Commercially Reasonable Efforts” shall mean continuous and diligent efforts of a degree and kind, including the level of attention and care and providing of funding and manpower, as are consistent with industry custom and practice and with the then current stage of product life cycle, which efforts shall in no event be less than the efforts that a Party applies with respect to its other programs and products of similar commercial potential consistent with the exercise of good business judgment for the maximization of profits.
1.9 “Confidential Information” shall mean any and all inventions, ideas, discoveries, data, instructions, designs, information, components, methods, tools, developments, innovations, techniques, materials, technology, protocols, procedures, results, formulae, trade secrets, know-how and other non-public and proprietary materials, products, processes or information, including research, product plans, manufacturing processes, manufacturing or operating costs, services, software, hardware, customer lists, price lists, business plans, marketing plans or financial information, that is or was disclosed or supplied by a Party (the “Disclosing Party”) to the other Party (the “Receiving Party”) in connection with this Agreement or the CDA. Disclosures by a Party’s Affiliate shall be deemed disclosures by that Party, and disclosures to a Party’s Affiliate shall be deemed disclosures to that Party.
Notwithstanding the foregoing, Confidential Information shall not include any part of the foregoing that the Receiving Party can prove:
1.9.1 Was already known to the Receiving Party as evidenced by the Receiving Party’s competent, contemporaneous written records, other than any portion of such information that was under an obligation of confidentiality at the time of its disclosure;
1.9.2 Became generally available to the public or otherwise becomes part of the public domain after disclosure of such information to the Receiving Party, other than by breach of this Agreement by the Receiving Party or by anyone to whom the Receiving Party disclosed such information;
1.9.3 Was subsequently lawfully without any restriction on disclosure disclosed to the Receiving Party by a Third Party other than in breach of a confidentiality obligation of such Third Party to the Disclosing Party; or
1.9.4 Was independently developed or discovered by employees of the Receiving Party who had no access to the Confidential Information of the Disclosing Party and did not make use of the Confidential Information of the Disclosing Party, as demonstrated by competent, contemporaneous written records.
1.10 “Controlled” or “Controls”, when used in reference to intellectual property, shall mean the legal authority or right of a Party (or any of its Affiliates) to grant a license or sublicense of intellectual property rights to the other Party, or to otherwise disclose proprietary or trade secret information to the other Party, without breaching the terms of any agreement with a Third Party, infringing upon the intellectual property rights of a Third Party, or misappropriating the proprietary or trade secret information of a Third Party. This term may be used herein as a noun.
| 3 |
Execution Copy
Confidential
1.11 “Data” shall mean any and all data from research and development work, including but not limited to all data from Clinical Studies or Non-Clinical Studies and regulatory submissions, related to the Ingredient or Product, including but not limited to data related to metabolites, degradation substances and impurities.
1.12 “Development Plan” shall mean the written document prepared and determined by SKK that describes the overall program for development of the Product in the Field in the Territory. The Development Plan shall include, among other things, estimated budgets, activities and timelines for pre-clinical studies and Clinical Studies for the Product, including toxicology, pharmacology and efficacy studies in humans, planned to be conducted to achieve each step towards procurement of Marketing Authorization. The Development Plan also shall forecast the initial Ingredient and/or Product supply requirements for such development activities.
1.13 “Existing Filing Document” shall mean the document(s) submitted by Can-Fite to FDA to enable Can-Fite to lawfully initiate a Phase II Clinical Trial (as defined below) with respect to the Product.
1.14 “FDA” shall mean the United States Food and Drug Administration, or any successor entity thereto.
1.15 “Field” shall mean systemic use (oral and injectable) of the Product for the therapeutic treatment of inflammatory diseases in humans; provided, however, that notwithstanding the foregoing, SKK shall not sell Product that is labeled for ophthalmic use.
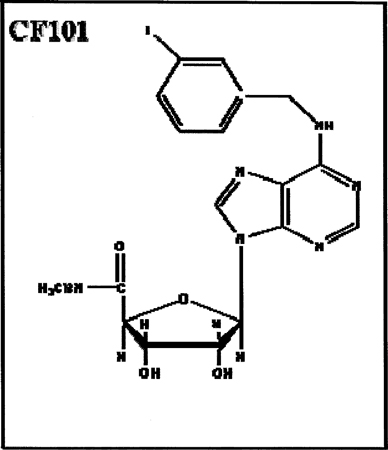
1.16 “Ingredient” shall mean an adenosine A3 receptor agonist designated by Can-Fite as CF101, and known generically as IB-MECA (Methyl 1-[N6-(3-iodobenzyl)-adenin-9-y1]-β-D-Ibofuronamid), the chemical structure of which is illustrated in Exhibit A.
1.17 “Knowledge” shall mean, with respect to a Party, the good faith understanding of the facts and information in the possession of an officer of such Party, or any in-house legal counsel of such Party, without any duty to conduct any additional investigation with respect to such facts and information by reason of the execution of this Agreement. For purposes of this definition, an “officer” shall mean any person in the position of senior vice president, president, chief operating officer or chief executive officer of a Party.
1.18 “Licensed Know-How” shall mean all ideas, data, instructions, discoveries, inventions, processes, formulae, techniques, procedures, designs, sketches, records, components, methods, tools, developments, innovations, materials, technology, protocols, results, expert opinions and other information Controlled by Can-Fite as of the Effective Date and during the term of this Agreement relating to the Ingredient and/or the Product that are not in the public domain and that are necessary for the development, use, manufacture (as authorized under this Agreement) or sale of the Ingredient and/or Product in the Territory. Licensed Know-How shall expressly exclude Licensed Patents.
| 4 |
Execution Copy
Confidential
1.19 “Licensed Patents” shall mean the patents and patent applications Controlled by Can-Fite as of the Effective Date and during the term of this Agreement relating to the Ingredient and/or the Product and/or the use of the Ingredient or the Product for treatment of a disease within the Field and having one or more Valid Claims within the Territory. The Licensed Patents are identified in Exhibit B, attached hereto and incorporated herein, as it may be amended by the Parties from time to time.
1.20 “Licensed Technology” shall mean the Licensed Know-How and the Licensed Patents.
1.21 “Manufacturing Cost” shall mean all costs for the Ingredient, calculated by using Can-Fite’s standard accounting procedures. Such costs shall include, but not be limited to, the fully burdened costs of all raw materials, labor and reasonable overhead for the synthesis, formulation, filling, finishing, labeling, packaging, storing, quality control and assurance activities and procurement costs associated with the Ingredient.
1.22 “Marketing Authorization” shall mean all approvals (including labeling, price and reimbursement approvals, if applicable), licenses, registrations or authorizations of any Regulatory Authority necessary for the commercial marketing, sale and use of the Product, as the case may be, in the Territory.
1.23 “MHLW” shall mean (i) the Ministry of Health, Labour and Welfare, the Japanese drug regulatory authority, and (ii) the Pharmaceuticals and Medical Devices Agency, an incorporated administrative agency who is the contractor of said Ministry (a) to provide guidance and advice on clinical trials, (b) to review and assess the Regulatory Filings, (c) to assess compliance with the GLP and GCP requirements, and to make GMP audits, and (d) to manage the safety and efficacy information during pre- and post-marketing phases, or any successor of their functions.
1.24 “NDA” or “New Drug Application” shall mean a new drug application filed with a Regulatory Authority, wherein NDA approval shall permit marketing of the applicable Product.
1.25 “Net Sales” shall mean the total amount invoiced to Third Parties in connection with sales of the Product by SKK, its Affiliates, its distributors and its Sublicensees to wholesalers or other Third-Party purchasers, less the following items to the extent actually paid or allowed and specified on any documents related to such sales:
1.25.1 Packaging, transportation and prepaid insurance charges on shipments or deliveries of Product;
1.25.2 Credit or refund actually allowed for any returned Product;
| 5 |
Execution Copy
Confidential
1.25.3 Reasonable and customary rebates, actually granted or given to wholesalers or other distributors; and
1.25.4 Sales or value added taxes actually incurred and paid by SKK, its Affiliates or Sublicensees in connection with the sale or delivery of the Product.
No deductions shall be made for cost of collections or for commissions paid to individuals, whether they be with independent sales agencies or regularly employed by SKK, and/or its Affiliates and on its or their payroll. Product shall be considered “sold” when billed out or invoiced. Sale or transfer to an Affiliate for resale by such Affiliate shall not be considered a sale for the purpose of this provision, but the resale by such Affiliate to a Third Party shall be a sale for such purpose.
No multiple royalties shall be payable to Can-Fite because the manufacture, use, sale, offer for sale or importation of any Product is covered by more than one of the Licensed Patents.
1.26 “Non-Clinical Study/Studies” shall mean any and all pre-clinical studies and non-clinical studies as may be required to be conducted and/or produced by or on behalf of either Party, and (if applicable) by Sublicensee(s) or Can-Fite’s Other Licensee(s), in connection with obtaining Marketing Authorization for the Product either inside or outside of the Territory. Non-Clinical Studies shall include any research and development conducted by either Party on the dosage form of the Product.
1.27 “Non-Clinical Study Costs” shall mean the entire costs (including reasonable overhead) relating to the performance of a Non-Clinical Study/Studies, including (i) payments made to CROs, laboratories, physicians, investigators, consultants and other personnel directly related to the performance of a Non-Clinical Study, (ii) costs of Ingredient and Product used in the Non-Clinical Study, (iii) costs associated with the preparation of a final report of the Non-Clinical Study, and (iv) reasonable internal costs relating to the Non-Clinical Study.
1.28 “Phase II Clinical Trial” shall mean a human clinical trial of the Product, the principal purpose of which is a determination of safety and efficacy of the Product in the target patient population, or a similar clinical study prescribed by the Regulatory Authority in the Territory. The term “Phase II Clinical Trial” shall expressly include both or either Phase IIa Clinical Trials and Phase IIb Clinical Trials. A Phase II Clinical Trial shall be deemed to have commenced when the first patient or subject in such study has been enrolled.
1.29 “Phase III Clinical Trial” shall mean a human clinical trial of the Product, on a sufficient number of subjects that is designed to establish that the Product is safe and efficacious for its intended use, and to determine warnings, precautions, and adverse reactions that are associated with the Ingredient or Product in the dosage range to be prescribed, which trial is intended to support Marketing Authorization for the Product, as the case may be. A Phase III Clinical Trial shall be deemed to have commenced when the first patient or subject in such study has been enrolled.
| 6 |
Execution Copy
Confidential
1.30 “Product” shall mean a pharmaceutical product intended for use or sale in the Field, wherein such product (i) contains the Ingredient as the active pharmaceutical ingredient, (ii) meets the applicable Specifications, and (iii) is in a form appropriate for systemic administration to a recipient.
1.31 “Regulatory Authority” shall mean, with respect to any particular country, territory or union, the governmental authority, body, commission, agency or other instrumentality of such country, territory or union with the primary responsibility for the evaluation or approval of pharmaceutical products before such pharmaceutical product may be tested, marketed, promoted, distributed or sold in such country, including such governmental bodies that have jurisdiction over the pricing of such pharmaceutical product. The term “Regulatory Authority” includes the MHLW, the FDA, and the European Agency for the Evaluation of Medicinal Products (“EMEA”).
1.32 “Regulatory Filing” shall mean all filings with the applicable Regulatory Authority for registrations, permits, licenses, authorizations, approvals, or notifications that are required to develop, make, use, sell, import or export the Product, as the case may be, and shall include a New Drug Application.
1.33 “Reimbursement Price” shall mean the price that may be charged for the Product in the Territory, as determined by the Regulatory Authority or the health authorities or any other authority that controls or regulates drug prices in the Territory.
1.34 “Sublicensee” shall mean an Affiliate of SKK or a Third Party to whom SKK has granted a right to manufacture, market, promote, distribute, and/or sell the Product (and/or to manufacture Ingredient, but only if SKK exercises its option to manufacture Ingredient in accordance with Section 7.7) within the Territory in accordance with Section 2.3. Notwithstanding the foregoing sentence, it is understood that, unless applicable laws and/or regulations require SKK to grant a sublicense to a Third Party distributor(s) of the Product in the Territory, who will be appointed by SKK for the specific purpose of marketing, promoting, distributing and/or selling Product in the Territory, such Third Party distributor(s) shall not be deemed to be a Sublicensee(s) for purposes of this definition.
1.35 “Territory” shall mean Japan.
1.36 “Third Party” shall mean any person or entity other than the Parties or their Affiliates.
1.37 “Trademarks” shall mean, as of the Effective Date and during the term of this Agreement, the Ingredient-specific and/or Product-specific trademarks that are used, or are intended to be used, by Can-Fite or SKK, or by any of their Affiliates or contractually bound Third Parties, in conjunction with distribution, promotion, marketing, sales, offers to sell, import, export or other exploitation of Product. The Trademarks licensed for use in the Territory are identified in Exhibit C, attached hereto and incorporated herein, as it may be amended by the Parties from time to time. All such Trademarks, whether in the English language or any other language, shall be owned by Can-Fite.
| 7 |
Execution Copy
Confidential
1.38 “Valid Claim” shall mean (i) a composition of matter claim, a method claim, a use claim, a pharmaceutical composition claim or an equivalent claim of an issued and unexpired patent (including a use patent) in the Territory covering the Ingredient, the Product or its pharmaceutical use, or (ii) a composition of matter claim, a method claim, a use claim, a pharmaceutical composition claim or an equivalent claim of a pending patent application in the Territory covering the Ingredient, the Product or its pharmaceutical use, but only if such claim within such pending patent application is being diligently prosecuted, and only if such claim has not been revoked or held unenforceable or invalid by a decision of a court or governmental agency of competent jurisdiction from which no appeal can be taken, or with respect to which an appeal is not taken within the time allowed for appeal, and that has not been disclaimed, denied or admitted to be invalid or unenforceable through reissue, disclaimer or otherwise, and that has not been lost through an interference proceeding or by abandonment.
1.39 Additional Definitions:
| Defined Term | Section in which Defined | |
| Actual Cost | 7.4.2 | |
| Agreement | Preamble | |
| Bankrupt Party | 14.3 | |
| Breaching Party | 14.2 | |
| Can-Fite | Preamble | |
| Can-Fite Indemnitees | 12.2 | |
| Can-Fite Invention | 10.2.2 | |
| Can-Fite’s Facility | 7.6.1 | |
| CGL | 12.4 | |
| CRAs | 1.6 | |
| CROs | 1.6 | |
| Disclosing Party | 1.9 | |
| Dispute | 15.1 | |
| Dosage Form Development | 5.1 | |
| Effective Date | Preamble | |
| EMEA | 1.31 | |
| ICC | 15.2 | |
| Indemnified Party | 12.3 |
| 8 |
Execution Copy
Confidential
| Defined Term | Section in which Defined | |
| Indemnifying Party | 12.3 | |
| Inventions | 10.2.1 | |
| Joint Committee or JC | 3.1 | |
| List of Can-Fite Studies | 4.1 | |
| Losses | 12.1 | |
| Manufacturing Process | 11.1 | |
| Marketing Plans | 8.2 | |
| Non-Breaching Party | 14.2 | |
| Parties | Preamble | |
| Party | Preamble | |
| Receiving Party | 1.9 | |
| Senior Executives | 15.1 | |
| SKK | Preamble | |
| SKK Indemnitees | 12.1 | |
| SKK Invention | 10.2.2 | |
| Specifications | 7.3 | |
| Supply Agreement | 7.3 | |
| Withholding Tax | 9.8 |
ARTICLE 2.
LICENSE
2.1 License Grant. Subject to the terms and conditions of this Agreement, Can-Fite hereby grants to SKK during the term of this Agreement a sole and exclusive license, even as against Can-Fite, under the Licensed Technology (i) to develop, import and use the Ingredient in the Field in the Territory, and (ii) to develop, have developed, register, market, have marketed, produce, have produced, distribute, have distributed, sell, have sold, offer for sale and import the Product in the Field in the Territory, and (iii) to have produced the Product outside the Territory for sale of such Product in the Field in the Territory. Such right granted to SKK pursuant to this Section 2.1 shall include SKK’s right under the Licensed Technology to conduct research on doses, formulations and dosage forms of the Product.
2.2 Trademark License. Subject to the terms and conditions of this Agreement, Can-Fite hereby grants to SKK an exclusive, royalty-free, fully paid-up license to use the Trademarks in connection with the distribution, marketing, promotion and sale of Product in the Field in the Territory, subject to quality control conditions established by Can-Fite, for so long as SKK is distributing, marketing, promoting and selling the Product in accordance with this Agreement. SKK is entitled to sublicense the Trademarks on a royalty-free basis within the above scope to Sublicensee(s).
| 9 |
Execution Copy
Confidential
2.3 Sublicenses. SKK shall have the right to grant sublicenses under the licenses set forth in Sections 2.1 and 2.2 to Sublicensees, subject to the following conditions: (i) the execution of an agreement between SKK and any Sublicensee shall not in any way diminish, reduce or eliminate any of SKK’s obligations under this Agreement, and SKK shall remain primarily liable for such obligations; (ii) SKK shall require each Sublicensee to agree in writing in its sublicense agreement to be bound by and comply with all the provisions and limitations of this Agreement applicable to SKK that are applicable to the rights sublicensed therein; (iii) SKK shall discuss such proposed sublicense with Can-Fite prior to SKK’s commitment to such Sublicensee; (iv) SKK shall provide Can-Fite a copy of any such proposed sublicense agreement (with financial and confidential information redacted); and (v) Can-Fite shall have approved the Sublicensee and the sublicense agreement in writing before the execution of any such sublicense, which approval shall not be unreasonably delayed or withheld. Without limiting the foregoing, SKK shall remain responsible to Can-Fite for payment of royalties due under this Agreement on the Net Sales of each such Sublicensee and for each Sublicensee’s adverse event reporting, pharmacovigilance and product complaint obligations under this Agreement. The permitted Sublicensees may not further sublicense any rights granted hereunder without the prior written consent of Can-Fite.
2.4 Right of Negotiation for Additional Exclusive License(s) within Asia. Upon SKK’s request at any time during the term of this Agreement, Can-Fite will enter into good faith negotiations for a period of ninety (90) days with SKK for the grant to SKK of an exclusive Product license(s) outside of the Territory but within Asia (excluding China and India) that may be requested by SKK. Upon mutual agreement between the Parties regarding the terms and conditions of such Product license, the Parties will enter into a separate license agreement therefor; provided, however, that Can-Fite shall not have any obligation to enter into such negotiations if Can-Fite is negotiating with or has entered into an agreement in respect of such Product license with a Third Party under the Licensed Technology for use in the Field in the particular country or territory which is the subject of SKK’s request; and provided further that, upon expiration of the above-mentioned ninety (90)-day negotiation period without a written agreement between the Parties, neither Party shall have any further obligation of any kind regarding such additional Product license(s).
2.5 Restrictions. During the term of this Agreement and as partial consideration for the licenses and rights granted hereunder, SKK shall not directly or indirectly, through one or more Affiliates or Third Parties, conduct, fund, license or participate in the development, distribution or commercialization in the Territory, in the Field, of any product containing an adenosine A3 receptor agonist as an active ingredient for use in the Field, other than the Product or as the Parties expressly agree in writing, regardless of whether such product is to be used for the same indication(s) as the Product. If SKK breaches its obligation under this Section 2.5, Can-Fite may convert the exclusive license granted in Section 2.1 to a non-exclusive license or may immediately terminate this Agreement, in Can-Fite’s sole discretion.
| 10 |
Execution Copy
Confidential
2.6 Retained Rights. Can-Fite retains all rights to research, develop, have developed, commercialize, use, market, have marketed, distribute, have distributed, sell, have sold, offer for sale, make, have made, import, export and otherwise exploit the Ingredient, the Product and the Licensed Technology outside the Field in the Territory and in the Field outside the Territory. For the sake of clarity, the exclusive license granted to SKK under Section 2.1 shall not preclude Can-Fite from conducting research with academic investigators in Japan. Subject to Section 7.7, Can-Fite shall have the sole and exclusive right (itself or through a Third Party) to manufacture or have manufactured the Ingredient and to supply the same to SKK as described herein.
2.7 No Implied Licenses. SKK acknowledges that the commercialization licenses granted by Can-Fite herein are limited to the Product in the Field in the Territory. No rights or licenses, including any research or development rights, with respect to products (other than the Product), the Licensed Technology or other intellectual property Controlled by Can-Fite are granted or shall be deemed granted hereunder or in connection herewith, other than those rights expressly granted in this Agreement.
ARTICLE 3.
JOINT COMMITTEE
3.1 Joint Committee. The Parties have a common understanding that it is necessary and desirous to harmonize and make consistent SKK’s activities related to the development of the Product in the Field in the Territory hereunder, and Can-Fite’s independent activities pertaining to development of the Product in the Field outside the Territory. To realize such harmonization and consistency, Can-Fite and SKK shall establish a joint committee (the “Joint Committee” or “JC”) to facilitate communication and coordination between the Parties in this regard. The Joint Committee shall facilitate the assistance provided by Can-Fite to SKK in order to achieve the mutually desired objective of speed, efficiency and coordination regarding SKK’s Product development activities hereunder. The Joint Committee’s responsibilities shall include review and discussion of: (i) the Development Plan, SKK’s progress with respect to the Development Plan’s activities and objectives, and the results and other outcomes of the development of the Product under the Development Plan in the Field; (ii) the strategic and operational issues identified by SKK in connection with Product development in the Field in the Territory by or on behalf of SKK; (iii) the plan and the protocols for pertinent Non-Clinical Studies and Clinical Studies to be conducted by or on behalf of Can-Fite with respect to the Product in the Field outside the Territory; (iv) Can-Fite’s general progress, results and other outcomes of development of Product in the Field outside the Territory; and (v) the strategic and operational issues identified by Can-Fite in connection with Product development in the Field outside the Territory by or on behalf of Can-Fite. Both Parties will freely and candidly exchange views and opinions, and offer advice, recommendations or suggestions to the other Party, in order to foster harmonization and consistency with respect to global Product development. Each Party shall respect and reasonably consider the other Party’s view, opinion, advice, recommendation and suggestion. The JC meetings may serve as a meeting of the Parties for information exchange purposes, as set forth herein. The Joint Committee shall cease to function, and this Article 3 shall have no further force and effect, upon the date that SKK is no longer pursuing clinical development (including post-marketing development and studies) of the Product in the Field in the Territory.
| 11 |
Execution Copy
Confidential
3.1.1 Membership. The JC shall be comprised of four (4) members, with two (2) members appointed by Can-Fite and two (2) members appointed by SKK. Each Party shall at all times have at least one (1) representative on the JC that is at a function head level. Each Party may replace one or more of its JC representatives at any time, with prior written notice to the other Party. With the consent of the JC members, other representatives of Can-Fite or SKK may attend JC meetings as non-voting observers.
3.1.2 JC Meetings. Except as otherwise expressly and mutually agreed by the Parties’ lead representatives on the JC, the JC shall meet at least once each calendar quarter, and at such other times and at places as are agreed to by both Parties. Half of the meetings shall take place in person; the other half may take place either in person or via tele-or video-conference. Each Party shall bear its own personnel and travel costs and expenses relating to JC meetings. Each Party’s lead representative shall co-chair meetings of the JC, and both co-chairs (or one of them, as may be agreed between them) shall be responsible for preparing the meeting agendas and minutes in turn. JC meeting minutes shall be distributed in draft form not later than thirty (30) days following each JC meeting, and shall be deemed accepted and effective unless an authorized representative of either Party has objected to the same in writing within thirty (30) days of the Parties’ receipt of such minutes. Final minutes of each JC meeting shall be promptly distributed to the Parties.
3.2 No Committee Amendments; Authority. Notwithstanding the creation of the JC, each Party to this Agreement shall retain the rights, powers, and discretion granted to it hereunder, and the JC shall not be delegated or vested with any such rights, powers, or discretion unless such delegation or vesting is expressly provided for herein or the Parties expressly so agree in writing. The JC shall have no power to amend or modify this Agreement, which may be amended or modified only as provided in Section 16.6.
ARTICLE 4.
EXCHANGE OF INFORMATION
4.1 Information Disclosure by Can-Fite Prior to the Effective Date. Prior to the Effective Date, Can-Fite has used Commercially Reasonable Efforts to disclose to SKK the Existing Filing Document and Licensed Technology. SKK acknowledges Can-Fite’s delivery, prior to the Effective Date, of a list indicating the title and study number of the Non-Clinical Studies, Clinical Studies and tests contained in the Existing Filing Document, as well as other Non-Clinical Studies, Clinical Studies and tests related to the Product that have been initiated as of the Effective Date (“List of Can-Fite Studies”). Such List of Can-Fite Studies includes a notation as to (i) whether or not such Non-Clinical Studies, Clinical Studies and/or tests were/ are being conducted in compliance with “Good Laboratory Practice” (for Non-Clinical Studies) and in compliance with “Good Clinical Practice” (for Clinical Studies), and (ii) whether such Non-Clinical Studies, Clinical Studies and/or tests were completed or are “on-going” (which indicates that a study or test has been initiated, but not yet been completed).
| 12 |
Execution Copy
Confidential
4.2 Disclosure of Intellectual Property by the Parties During the Term. During the term of this Agreement, Can-Fite shall use Commercially Reasonable Efforts to disclose to SKK Licensed Technology that is necessary to SKK’s full enjoyment of the license rights granted to SKK hereunder. During the term of this Agreement, SKK shall use Commercially Reasonable Efforts to disclose to Can-Fite intellectual property (including patent rights and know-how) that is necessary to Can-Fite’s full enjoyment of its retained rights hereunder.
4.3 Information Exchange. In addition to disclosure to the Joint Committee of the progress and results of pertinent Non-Clinical Studies and Clinical Studies regarding the Product which were not disclosed prior to the Effective Date, each of Can-Fite and SKK shall provide to the other summary reports generated in the conduct of pertinent Clinical Studies and Non-Clinical Studies of the Product, as well as written summaries of the Regulatory Filings regarding the Product, upon completion of each phase of such Clinical Studies or completion of tests within such Non-Clinical Studies; in all cases subject to Third-Party confidentiality restrictions as may exist. All such Product-related information exchanged hereunder (including such summary reports and written summaries, which shall include sufficient information to enable the recipient to understand each study and its results) shall be written in the English language. In addition, upon reasonable request by a Party in writing in advance, the other Party shall provide access at its facility(ies) to the extent necessary to enable the requesting Party to review on-site the study-specific portions of detailed Product-related analyses, Data, written Product-related reports, and Regulatory Filings that are made a part of, are related to, or are quoted in such summary reports or such written summaries. Except as provided in the following sentences of this Section 4.3, the requesting Party shall not make or remove any copies of any documentation to which the requesting Party was given access. Any out-of-pocket costs that are incurred by the Party granting such access to the requesting Party shall be fully reimbursed by the requesting Party promptly after receipt of invoice(s) for such out-of-pocket costs. If the requesting Party decides that it wishes to obtain a copy of the full report regarding such Clinical Studies, Non-Clinical Studies and/or Regulatory Filings of the Product, the requesting Party shall provide written notice of such decision to the other Party. The Parties will discuss the manner in which such full report copy will be produced and provided to the requesting Party, at the requesting Party’s sole expense (and such provision of a full copy is subject to the providing Party’s prior receipt of the cost-sharing payment(s) and amounts set forth in Section 9.3 or 9.4, as applicable). Subject to the terms and conditions of this Agreement (including Sections 9.3 and 9.4), after receipt of such full report copy the requesting Party may (i) reprint such Product-related analyses, Data, Product-related reports and Regulatory Filings of the other Party for use and/or incorporation into Product Regulatory Filings of the requesting Party; and (ii) quote or describe data and information contained in such Product-related analyses, Data, Product-related reports and Regulatory Filings of the other Party in Product Regulatory Filings of the requesting Party; in all cases subject to Third-Party confidentiality restrictions as may exist; provided, however, that SKK’s right to receive and use such full report and portions thereof (for example, analyses, Data, reports and Regulatory Filings of Can-Fite) shall be contingent on SKK’s payment of Clinical Study Costs and Non-Clinical Study Costs and other amounts set forth in Section 9.3, and Can-Fite’s right to receive and use such full report and portions thereof (for example, analyses, Data, reports and Regulatory Filings of SKK) shall be contingent on Can-Fite’s payment of Clinical Study Costs and Non-Clinical Study Costs and other amounts set forth in Section 9.4. In addition to the foregoing, and to the extent permitted by Third Party confidentiality obligations and applicable laws and regulations, each Party shall use Commercially Reasonable Efforts to disclose to the Joint Committee in good faith any findings of which it becomes aware regarding adenosine receptor expression in humans, and each Party may use such findings regarding adenosine receptor expression in humans to support Product Regulatory Filings, and for marketing and other commercialization activities pertaining to the Product.
| 13 |
Execution Copy
Confidential
4.4 Can-Fite’s Other Licensee(s). If Can-Fite’s Other Licensee(s) conducts Non-Clinical Studies in the Field outside of the Territory, then with respect to Data obtained in such Non-Clinical Studies only, Can-Fite agrees to cause Can-Fite’s Other Licensee(s) to accept the conditions provided in this Article 4 and to undertake to disclose such Data to SKK directly or through Can-Fite. If Can-Fite possesses and Controls information and/or Data obtained from Can-Fite’s Other Licensee(s) regarding Clinical Studies performed by Can-Fite’s Other Licensee(s) in the Field outside of the Territory, then to the extent that Can-Fite has the right, under its contractual agreement(s) with such Can-Fite’s Other Licensee(s), to disclose such information and/or Data to SKK, Can-Fite will disclose to SKK such information and/or Data of Can-Fite’s Other Licensee(s). Can-Fite will use good faith efforts to include in its agreements with Can-Fite’s Other Licensee(s) the right to disclose Data to SKK and to grant a right to SKK to incorporate the same into the Regulatory Filing that Can-Fite obtains from Can-Fite’s Other Licensee(s). If, after using such good faith efforts, Can-Fite does not have the right to disclose to SKK the Data obtained from such Can-Fite’s Other Licensee(s), then Can-Fite will use good faith efforts to facilitate a direct interaction between SKK and such Can-Fite’s Other Licensee(s), so that SKK may seek to obtain such Data directly from such Can-Fite’s Other Licensee(s).
ARTICLE 5.
DEVELOPMENT; REGULATORY
5.1 Dosage Form Development. Can-Fite will conduct and complete research and development on change of the dosage form of the Product from capsule to tablet (“Dosage Form Development”) six (6) months prior to commencement of Phase I Clinical Trial by SKK; as of the Effective Date, the anticipated date for such commencement by SKK is July 1, 2008. Can-Fite will invite SKK’s input on Dosage Form Development, and will use good faith efforts to meet the needs of SKK in this regard, but Can-Fite shall have final decision-making authority regarding the tablet dosage form of the Product to be used outside of the Territory. The results of Dosage Form Development shall be disclosed to SKK promptly after completion of Dosage Form Development in writing and incorporated into the Licensed Technology or Data respectively.
| 14 |
Execution Copy
Confidential
5.2 Development Plan. SKK understands and agrees that the Development Plan may not contain elements that materially and adversely affect, or may otherwise have the effect of materially and adversely affecting, Can-Fite’s ability to conduct development, commercialization or other exploitation of the Ingredient and the Product outside of the Field and/or outside the Territory. Based on the above, SKK shall prepare the final draft of the Development Plan and submit it to Can-Fite for review promptly after its preparation. The Development Plan shall set forth in reasonable detail SKK’s development activities to be conducted to develop the Product and receive Marketing Authorization in the Field in the Territory. Such review of and comment on the draft Development Plan will be conducted by Can-Fite in good faith. SKK shall respect and take into consideration the views, opinions, advice, recommendations and/or suggestions advanced by Can-Fite with respect to the draft Development Plan, and, if necessary, if Can-Fite’s proposed revisions were given timely, and if SKK accepts the revisions proposed by Can-Fite, SKK will incorporate such revisions into the Development Plan; provided, however, SKK shall have the sole and exclusive discretion to finalize the Development Plan. Subject to the first sentence of this Section 5.2 and the other terms and conditions of this Agreement, SKK may modify or add any test or study within the finalized Development Plan at its sole discretion, upon prompt notification to Can-Fite. Notwithstanding anything to the contrary herein, SKK shall have the sole and exclusive discretion and decision-making authority to determine whether or not to employ the Bridging Strategy in the development of the Product in the Field in the Territory and, if SKK determines that the Bridging Strategy will be employed, SKK shall have the exclusive right to conduct such Bridging Strategy in the Field in the Territory using the Data disclosed by Can-Fite hereunder.
5.3 Protocol of Non-Clinical Studies by Can-Fite. Can-Fite shall make the draft protocols for the Non-Clinical Studies conducted by or on behalf of Can-Fite available to SKK in the English language for review and comment by SKK. SKK shall deliver its comments (if any) to Can-Fite within fifteen (15) days after SKK’s receipt of the draft protocols, which comments Can-Fite shall take into account in good faith in finalizing such protocols, but Can-Fite is entitled to finalize such protocols at its sole discretion.
5.4 Development Conduct and Costs. SKK shall be responsible for conducting all development activities under the Development Plan, including submission of all Regulatory Filings for the Product in the Territory and all Clinical Studies in the Territory under the Development Plan, if the results of such Clinical Studies support such Regulatory Filing submission, in SKK’s judgment. SKK shall, subject to Section 9.4, bear all costs it incurs in conducting such development, including expenses SKK incurs in conducting Clinical Studies and in preparing for the same, as well for all regulatory activities in the Territory, including preparation of regulatory documents or any supplemental studies necessary to achieve Marketing Authorization for the Product in the Territory. Prior to initiation by SKK, the protocols of all Clinical Studies and Non-Clinical Studies shall be submitted to Can-Fite for review and comment by Can-Fite. Such review and comment regarding the protocols of all Clinical Studies and the related Non-Clinical Studies will be conducted by Can-Fite in good faith, and Can-Fite’s comments regarding such protocols and Non-Clinical Studies (as applicable) shall be respected and reasonably considered by SKK. SKK agrees to use its Commercially Reasonable Efforts to submit Regulatory Filings and obtain Marketing Authorization for the Product as soon as possible in accordance with the Development Plan.
| 15 |
Execution Copy
Confidential
5.5 Failure to Develop. Should SKK fail to proceed with development of the Product in accordance with the Development Plan, and/or if SKK has not submitted a Regulatory Filing for Marketing Authorization of the Product in the Field in the Territory within twelve (12) months after the date specified for such filing in the Development Plan (as it may be amended from time to time), other than for good faith reasons, such as but not limited to force majeure (as described in Section 16.1), Can-Fite will have the right (either itself or through a Third Party), exercisable upon written notice to SKK following the expiration of a ninety (90)-day cure period (or, if it is not practicable to complete the cure of such failure within such 90-day period, following the expiration of an extended period of time to be determined upon mutual written agreement of the Parties), to develop the Product (either itself or through a Third Party) in the Territory, and thereafter all rights to develop and commercialize the Product in the Territory shall revert to Can-Fite. This Section 5.5 shall not limit any other remedies Can-Fite may have under this Agreement or applicable law. Notwithstanding the foregoing provisions of this Section 5.5, Can-Fite is not entitled to forward the aforementioned notice to SKK, or, if forwarded by Can-Fite, such notice shall have no effect and force as specified above, in the following instances:
| (i) | If such failure was caused solely by an act or omission of Can-Fite or a Third Party contracted or designated by Can-Fite in connection with this Agreement; |
| (ii) | If such failure was noticed by SKK to Can-Fite in writing in a timely manner, together with a written plan for SKK’s practicably prompt cure or recovery, and such plan is accepted by Can-Fite in writing; provided that such acceptance of such plan by Can-Fite shall not be unreasonably withheld; and provided further that if SKK fails to achieve such cure or recovery in accordance with such plan, Can-Fite may deliver the aforementioned notice to SKK; |
| (iii) | If such failure was reasonably attributed to a lack of clinical efficacy and/or safety with respect to a Product, and SKK provides a written plan for continued development of such Product; or |
| (iv) | If such failure was caused by or resulted from events beyond the reasonable control of SKK, including but not limited to enactment, revision or repeal of a law, regulation, rule, guideline or the like, and/or a decree, order, instruction, guidance, warning or the like of the relevant Regulatory Authority or a court having jurisdiction, wherein such event precludes SKK from developing or obtaining Marketing Authorization for the Product as it is then configured; provided that SKK will prepare and provide to Can-Fite SKK’s written plan regarding other, lawful means whereby SKK would be likely to obtain Marketing Authorization for the Product within reasonable time. |
| 16 |
Execution Copy
Confidential
5.6 Reference Rights; Information and Data Used for Regulatory Purposes. Each Party shall have the right to refer to and cross reference, in their respective territories, regulatory dossiers and filings of the other Party pertaining to the Product (and to the extent permitted and applicable, regulatory dossiers and filings of Can-Fite’s Other Licensee(s) and/or Sublicensee(s)), for the purpose of supporting Regulatory Filings for the Product in the Field (such right includes a right to incorporate the summary received pursuant to Section 4.3 into the Regulatory Filings), and to receive a written right of reference thereto for filing with Regulatory Authorities free of charge. Subject, among other things, to the provisions of Sections 4.3, 9.3 and 9.4, as applicable, each Party will be entitled to receive, keep and use for regulatory purposes (i) information and Data pertaining to the Product in the Field provided by the other Party pursuant to Article 4 in the form of full copy of the report regarding the relevant Clinical Studies, Non-Clinical Studies or Regulatory Filings, and (ii) to the extent required by applicable Regulatory Authorities and/or applicable laws, rules and regulations in each Party’s respective territory, other documents relating to the Product in the Field filed by the other Party with Regulatory Authorities in its territory, and any written communications to and with any Regulatory Authority by the other Party pertaining to the Product in the Field, and other findings and information additionally provided pursuant to Article 4; provided that any out-of-pocket expenses incurred by the providing Party related to the provision of copies of such information, Data or documents shall be borne by the accessing Party.
5.7 Manufacturing Documents. In order to help preserve the proprietary nature of Can-Fite’s manufacturing information relating to the Ingredient and/or the Product (e.g., the respective CMC section contained in any Regulatory Filings), Can-Fite will have the right, to the extent permitted by Regulatory Authorities, to file a drug master file with a Regulatory Authority to make the information regarding such manufacturing information available directly to the Regulatory Authority; provided, however, for the Territory, SKK will have the right to access and reference the drug master file registration number in its Regulatory Filing for the Product, including said CMC section and documentation, to the extent required by law, rule, regulation or a Regulatory Authority having jurisdiction in the Territory. Notwithstanding anything to the contrary herein, SKK will only be entitled to use the manufacturing information relating to the Ingredient, to the extent reasonably required by local or national law, rule, regulation or Regulatory Authority and to carry out its development and commercialization activities hereunder. If SKK exercises its option to manufacture the Ingredient in accordance with Section 7.7, SKK’s use of Can-Fite’s proprietary manufacturing information after such exercise of such option shall be mutually agreed by the Parties in writing.
5.8 Regulatory Filings. The harmonization and coordination of Regulatory Filings for the Product by both Parties shall be discussed at the JC. SKK shall make a summary report of each draft Regulatory Filing (wherein such summary report will include sufficient information to enable Can-Fite to understand the studies and results contained therein; however, its content shall be discussed and agreed at the JC) available to Can-Fite with English translation thirty (30) days prior to the meeting with the MHLW to be held in advance of the submission thereof to the MHLW, for review and comment by Can-Fite within fifteen (15) days after Can-Fite’s receipt of such summary report, which comments SKK shall take into account in good faith in finalizing such Regulatory Filing submission, but SKK is entitled to finalize it at its sole discretion. If SKK should make any material changes to such draft Regulatory Filing in producing the final Regulatory Filing, then, SKK shall inform Can-Fite of all such material changes as soon as practicable. All Regulatory Filings filed by SKK in the Territory shall be in the name of and owned by SKK, except those facility descriptions equivalent to those customarily found in a MHLW application relating to manufacturing of the Ingredient, which is owned by Can-Fite or its designee. SKK shall promptly notify Can-Fite in writing upon receiving Marketing Authorization in the Territory for the Product. When Can-Fite determines the anticipated date when Can-Fite will submit a Product Regulatory Filing to the Regulatory Authority outside the Territory, Can-Fite shall provide advance written notice to SKK informing SKK of such anticipated date of submission.
| 17 |
Execution Copy
Confidential
5.9 Regulatory Communications. SKK shall inform Can-Fite of the outline of all discussion and development at any and all meetings between SKK (or its designee) and Regulatory Authorities related to the Product. If and to the extent that discussions and/or developments at meetings between Can-Fite (or its designee) and Regulatory Authorities related to the Product should have a material impact on SKK’s development of Product in the Field in the Territory, Can-Fite shall inform SKK of the outline of such portions of such discussions and developments which result in such material impact.
5.10 Product Complaints, Pharmacovigilance and Adverse Event Reporting. Prior to commencement by SKK of the first Clinical Study of the Product in the Field in the Territory, the Parties shall discuss and agree upon a written standard operating procedure for reporting any adverse events and Product complaints, and for coordinating the collection, investigation, reporting, and exchange of information concerning any such adverse events or complaints. Such procedure shall be sufficient to permit each Party to comply with all applicable laws, regulations and guidelines and with its internal pharmacovigilance practices. The standard operating procedure will be promptly updated if required by changes in legal requirements. Each Party shall ensure that its Affiliates, Can-Fite’s Other Licensee(s) and Sublicensees comply with the standard operating procedure (or an equivalent procedure). Each Party will designate a liaison to be responsible for communicating with the other Party regarding the reporting of adverse events and complaints in connection with the Product. Information and/or Data pertaining to adverse events and/or safety data that are obtained from any Clinical Studies and Non-Clinical Studies performed by a Party shall be provided to the applicable Regulatory Authority, and promptly thereafter to the other Party; provided that the content of such disclosure to the other Party shall be the same as that provided to the applicable Regulatory Authority, as required by applicable regulatory requirements. The Parties will share any resultant regulatory action plans that may result therefrom. All adverse event reports and other safety data and information shall be provided to the other Party in English. Notwithstanding anything to the contrary in Section 4.3, the Parties will comply with all mandatory reporting requirements regarding safety data and adverse event reporting.
5.11 Compliance with Laws and Regulatory Requirements. SKK shall be responsible for ensuring that all Third Parties, Affiliates, and Sublicensees which manufacture, purchase, distribute or otherwise transfer the Ingredient and/or Product comply with the requirements of this Agreement and any and all requirements of the Regulatory Authorities regarding the Product including the development and/or commercialization of the Product. Each Party agrees to promptly inform the other Party of all MHLW, FDA or other Regulatory Authority regulations, notices, circulars or warnings applicable to the Product of which it becomes aware. Each Party shall perform its obligations under this Agreement and in the case of SKK, its responsibilities and rights under the Development Plan in connection with the development and commercialization of the Product in accordance with all applicable laws, rules and regulations, including those of all Regulatory Authorities in the Territory, applicable reporting obligations, and applicable import and export laws and regulations.
| 18 |
Execution Copy
Confidential
5.12 Applications for Regulatory Exclusivity. The Parties recognize the commercial value of exclusivity rights to Product granted or provided for under laws and regulations in the Territory. To the extent permitted by law, SKK will have the exclusive right to file for, request and maintain any regulatory exclusivity rights for Product in the Territory (including regulatory exclusivity rights based upon an orphan drug designation of Product) and to conduct and prosecute any proceedings or actions to enforce the regulatory exclusivity rights.
5.13 Protocols and Regulatory Communications Obtained from Can-Fite’s Other Licensee(s). If Can-Fite possesses and Controls any protocols for Non-Clinical Studies or pertinent regulatory communications obtained from Can-Fite’s Other Licensee(s) in the Field outside of the Territory, then to the extent that Can-Fite has the right, under its contractual agreement(s) with such Can-Fite’s Other Licensee(s), to disclose such protocols and/or regulatory communications to SKK, Can-Fite will disclose to SKK such protocols and/or regulatory communications of Can-Fite’s Other Licensee(s). Can-Fite will use good faith efforts to include in its agreements with Can-Fite’s Other Licensee(s) the right to disclose protocols and regulatory communications to SKK that Can-Fite obtains from Can-Fite’s Other Licensee(s). If, after using such good faith efforts, Can-Fite does not have the right to disclose to SKK the protocols and regulatory communications obtained from such Can-Fite’s Other Licensee(s), then Can-Fite will use good faith efforts to facilitate a direct interaction between SKK and such Can-Fite’s Other Licensee(s), so that SKK may seek to obtain such protocols and/or regulatory communications directly from such Can-Fite’s Other Licensee(s).
ARTICLE 6.
LABELING; TRADEMARKS
6.1 Labeling. SKK shall be responsible for the labeling of the Product in the Territory and for ensuring that such labeling is in compliance with all applicable laws in the Territory and rules and regulations of all Regulatory Authorities in the Territory.
6.2 Trademarks. Can-Fite shall be responsible for filing, registering and maintaining worldwide Trademarks for the Product, including in the Territory. Can-Fite will consult with SKK regarding the selection and registration of the Trademarks within the Territory. Can-Fite will register SKK as a registered user of the Trademarks, if required under the applicable law in the Territory.
| 19 |
Execution Copy
Confidential
6.3 Display. All packaging materials, labels, inserts and promotional materials for the Product sold in the Territory shall display: (i) the Trademarks, (ii) the trade name of SKK in the context of the Product as manufactured and distributed by SKK, and (iii) the trade name of Can-Fite in the context of the Product as manufactured by or for Can-Fite (whether in English or in the local language). The manner of use of the Trademarks, including typeface and size, representations of the Trademarks, as well as promotional material bearing the Trademarks, will be jointly agreed by the Parties. If a given Trademark is not applicable in the Territory, other trademarks, which shall be mutually approved by the Parties, shall be displayed on the label of the Product in the Territory. All representations of the Trademarks that SKK intends to use shall first be submitted to Can-Fite for approval of design, color, and other details or shall be exact copies of those used by Can-Fite, and shall in any event comply with Can-Fite’s usage and quality control guidelines as established from time to time. SKK shall submit representative promotional materials, packaging, labels and the Product using any Trademarks to Can-Fite for Can-Fite’s review and comment prior to their first use and prior to any subsequent change or addition to such materials. All approvals to be required under this Article 6 shall not be unreasonably withheld or delayed.
6.4 Ownership. SKK acknowledges that: (i) the Trademarks are owned exclusively by Can-Fite; (ii) that SKK has no right, title or interest in and to the Trademarks, except the rights conferred by this Agreement; and (iii) that all goodwill associated with the Trademarks vests in and inures to the benefit of Can-Fite. In acknowledgement of Can-Fite’s exclusive ownership rights in the Trademarks, SKK agrees at no time during or after the term of this Agreement to challenge or assist others to challenge the Trademarks or the registration thereof or attempt to register any trademarks, marks or trade names confusingly similar to any Trademarks for the use in pharmaceutical products. SKK’s use of the Trademarks shall inure to the benefit of Can-Fite.
6.5 Termination of Use of Trademarks. Upon termination of this Agreement, SKK shall discontinue all use of the Trademarks, terminate all sublicenses to the Trademarks and shall not thereafter adopt or attempt to register a mark that is confusingly similar to any of the Trademarks for the use in pharmaceutical products; provided, however, that upon expiration of this Agreement and SKK’s payment of all royalty amounts due under this Agreement, SKK’s and its Sublicensee(s)’ right to use the Trademarks in conjunction with the Product shall be converted to a paid-up license.
ARTICLE 7.
MANUFACTURE AND SUPPLY OF INGREDIENT
7.1 Generally. Subject to the terms and conditions of this Article 7 and a separate Supply Agreement for the Ingredient to be negotiated by the Parties, Can-Fite shall supply SKK (and through SKK, shall supply SKK’s Sublicensees) with all of their requirements for the Ingredient. Subject to Section 7.7, Can-Fite shall be SKK’s (and its Affiliates’ and Sublicensees’) exclusive supplier of the Ingredient during the term of this Agreement hereunder. It is understood that, subject to Section 7.7, SKK shall not have the right to manufacture, or to authorize any Affiliate, any Sublicensee or other Third Party to manufacture, the Ingredient, except as may be expressly provided in the Supply Agreement. For the sake of clarity, Can-Fite shall not sell Ingredient to any Third Party in the Territory.
| 20 |
Execution Copy
Confidential
7.2 Supply for Development Activities.
7.2.1 Obligations of the Parties. Can-Fite shall use Commercially Reasonable Efforts to timely supply the Ingredient, at SKK’s option, to SKK as necessary for SKK to carry out development, including Clinical Studies and Non-Clinical Studies (as applicable), of the Product in the Field in the Territory in accordance with the Development Plan. The Ingredient supplied to SKK for development, including incorporation into Product for Clinical Studies and Non-Clinical Studies (as applicable), in the Territory shall be supplied by Can-Fite to SKK in accordance with the form, quantities and schedule to be agreed upon in writing by the Parties. SKK shall present to Can-Fite its Ingredient supply requirements for Clinical Studies or Non-Clinical Studies (as applicable) in good time prior to initiating such studies, and Can-Fite will supply the Ingredient accordingly. SKK shall not sell Ingredient supplied under this Section 7.2 to a Third Party for commercial purposes. The terms and conditions for the Ingredient Supply Agreement not provided herein, but necessary for the supply of the Ingredient for development purposes, shall be negotiated between the Parties as soon as practicably possible.
7.2.2 Third Party Manufacturer of Product. Can-Fite shall use Commercially Reasonable Efforts to facilitate SKK in establishing a relationship with Can-Fite’s Third Party manufacturer of Product, with the objective that SKK would establish a direct relationship with such Third Party manufacturer of Product in respect of procurement of Product from such manufacturer. In the event that SKK establishes a direct relationship with such Third Party manufacturer, Can-Fite will assist SKK in management of its Product supply process and arrangements concerning such Third Party manufacturer.
7.3 Commercial Supply of the Ingredient. After the completion of the Phase II Clinical Trial of the Product in the Territory, the Parties shall negotiate in good faith and finalize the terms of a manufacturing, supply and quality agreement for commercial supply to SKK (and through SKK, to SKK’s Sublicensee(s)) of Ingredient, which shall set forth the terms and conditions set forth in this Article 7, and other mutually acceptable terms and conditions not inconsistent with this Agreement, including representations, warranties, limitations of liability and indemnities of the type and scope customary in the industry (the “Supply Agreement”). During the course of such negotiations, the Parties shall agree upon written specifications for the Ingredient (“Specifications”) which shall be attached to and incorporated in the Supply Agreement. Among other items, the Supply Agreement will include the following provisions:
7.3.1 Supply Agreement. Can-Fite will supply SKK with Ingredient in accordance with such forecasting and other supply requirements as are set forth in the Supply Agreement. Can-Fite may select a contract manufacturer to manufacture the Ingredient for SKK and its Affiliates and its Sublicensees under the Supply Agreement. All Ingredient manufactured by Can-Fite or its contract manufacturers for SKK under the Supply Agreement will be manufactured in accordance with the Specifications (which will include reference to the then-current good manufacturing practices under the rules and regulations of the FDA or such other rules as updated by ICH GMP Guidelines and regulations in the Territory).
| 21 |
Execution Copy
Confidential
7.3.2 Can-Fite’s Rights and Obligations. Except as otherwise provided herein, Can-Fite will have the right to make all decisions with respect to manufacturing in its sole discretion, including decisions relating to process development and manufacturing procedures, work to support quality control and quality assurance, improving manufacturing/cost efficiency and commercial scale-up manufacturing; provided that Can-Fite will manufacture or have the Ingredient manufactured in conformity with the Specifications and all applicable laws and regulations in the Territory. Can-Fite shall timely notify SKK of any manufacturing change that may have an impact on SKK’s ability to timely receive Marketing Authorization or jeopardize the current status of the Product in the Territory.
7.3.3 SKK’s Rights and Obligations. Unless otherwise agreed by the Parties, SKK will have final decision-making authority to fulfill all regulatory responsibilities over all subsequent steps of the Product manufacturing process that incorporate Ingredient into Product in the Territory (including finish and fill, labeling and packaging, lot release, and management of permitted subcontractors).
7.3.4 Other Terms and Conditions. The Supply Agreement will also set forth all other terms and conditions applicable to the manufacture, distribution, forecast, acceptance, rejection, supply, delivery, quality testing, quality control and quality assurance, third party liabilities, record keeping, audit and the like of Ingredient provided to SKK by Can-Fite.
7.4 Transfer Price; Taxes; Shipping.
7.4.1 Transfer Price for Development Purposes. The transfer price payable by SKK to Can-Fite for quantities of the Ingredient to be used for development purposes, including Clinical Studies and Non-Clinical Studies using the Product, shall be equal to Can-Fite’s Manufacturing Cost for such quantities of Ingredient plus transportation costs incurred by Can-Fite in connection therewith.
| 22 |
Execution Copy
Confidential
7.4.2 Transfer Price for Commercial Purposes. The transfer price payable by SKK to Can-Fite for quantities of the Ingredient to be incorporated into the Product and used for the sale, promotion, marketing, distribution or other commercialization of Product in the Territory shall be set at a price equal to seven percent (7%) of the Reimbursement Price for the Product; provided that, in no event shall the transfer price of the Ingredient calculated under this Section 7.4.2 be less than the actual Manufacturing Cost that corresponds to the final packaged unit of such Product (“Actual Cost”). If the Actual Cost exceeds seven percent (7%) of the Reimbursement Price for the Product, then SKK may elect from the following alternatives: (i) to purchase the Ingredient from Can-Fite at the Actual Cost, or (ii) to obtain a right to manufacture the Ingredient as provided in Section 7.7 without paying the option exercise fee of One Million U.S. Dollars ($1,000,000), but subject to the royalty payment pursuant to Section 7.7.2. Prior to Commercial Launch, SKK shall and can purchase, at Can-Fite’s Manufacturing Cost plus transportation costs, quantities of the Ingredient to be incorporated into the Product intended for Commercial Launch (plus Product intended for sale for a reasonable period of time thereafter). All Product produced from such pre-Commercial Launch quantities of Ingredient shall be sold first. To avoid double payments by SKK under Section 7.5.2, SKK shall document the units of Products sold that were produced from such pre-Commercial Launch quantities of Ingredient purchased at Can-Fite’s Manufacturing Cost plus transportation costs. With respect to the total of such commercialized Products so produced, the difference between the calculation set forth in Section 7.5.2 and the purchase price of such pre-Commercial Launch quantities of Ingredient incorporated into such commercialized Product will be determined and paid to Can-Fite.
7.4.3 Delivery of Ingredient. All Ingredient, whether for development or commercial purposes, shall be deemed to be delivered to SKK (or to SKK’s designee) at the point where Can-Fite delivers such Ingredient to the carrier selected by SKK, and the title and risk thereto shall be simultaneously transferred to SKK. SKK shall be responsible for all costs of transportation, freight, insurance, customs and import formalities pertaining to shipment of Ingredient to SKK (or to SKK’s designee).
7.5 Payments. Payments due to Can-Fite under Section 7.4 above shall be made in accordance with the applicable provisions of Sections 9.6 through 9.10, and a more specific payment method shall be provided in the Supply Agreement.
7.5.1 Development Supply. Can-Fite shall transmit to SKK an invoice detailing the Manufacturing Cost for the Ingredient delivered to SKK (or to SKK’s designee) hereunder for development purposes, including Non-Clinical Studies and Clinical Studies, and SKK shall make payment to Can-Fite within thirty (30) days after receipt of each such invoice.
7.5.2 Commercial Supply; Calculation of Ingredient Price. SKK shall forecast its projected Product sales in the Territory on a quarterly basis. The Parties will determine a reasonable and practicable mechanism for the payment of the price of the Ingredient by SKK to Can-Fite, which will be provided in the Supply Agreement. Unless otherwise agreed by the Parties in the Supply Agreement, the price for Ingredient shall be seven percent (7%) of the Reimbursement Price in effect at the time of SKK’s order, calculated as follows:
| (i) | [Number of kilograms of Ingredient ordered by SKK] times [#] = Anticipated Product Unit Equivalents; then |
| (ii) | [Anticipated Product Unit Equivalents] times [Reimbursement Price] times [7%] = price for Ingredient ordered by SKK, |
| 23 |
Execution Copy
Confidential
where “[#]” represents the net number of Product units manufactured for commercialization in the Territory that are derived from a kilogram of Ingredient, wherein such net number of Product units (i) shall be mutually agreed by the Parties prior to the first order by SKK pursuant to this Section 7.5.2, and (ii) shall be based initially upon historical production data provided by Can-Fite, taking into account the requirements for manufacture of Product for the Territory and commercialization of Product in the Territory, and (iii) may be revised from time-to-time upon written mutual agreement of the Parties, based on the actual Product production results obtained by or on behalf of SKK, and
where “Reimbursement Price” shall be the then-current Reimbursement Price at the time the order is placed by SKK, except that if the Reimbursement Price is not yet finalized (i.e., at the time of the initial SKK orders), then the Reimbursement Price shall be based on SKK’s good faith estimate. The Supply Agreement will provide for an appropriate adjustment if the actual Reimbursement Price (at the time that the Product is first sold in the Territory) differs from this good faith estimate.
7.6 Other Terms and Conditions for Supply Agreement.
7.6.1 Warranty. Can-Fite shall warrant that all Ingredient manufactured by it or Can-Fite’s Third Party manufacturer(s) and supplied to SKK shall be manufactured, stored and otherwise handled in compliance with the applicable ICH and Japanese governmental requirements; provided that SKK shall be fully responsible for informing Can-Fite of all such applicable Japanese governmental requirements. Further Can-Fite warrants that the production facility of Can-Fite and Can-Fite’s Third Party manufacturer(s) used for manufacturing the Ingredient supplied to SKK (jointly or separately, “Can-Fite’s Facility”) shall comply with all applicable laws and other regulatory requirements, including but not limited to cGMP and GMP.
7.6.2 Audit by SKK. During the term in which Can-Fite supplies the Ingredient to SKK, and one time prior to the commencement of such Ingredient supply as well, SKK is entitled to audit Can-Fite’s facility (and if Can-Fite is using a Third Party manufacturer to produce the Ingredient, SKK is entitled to require Can-Fite to audit such Third Party manufacturer’s facility on behalf of SKK), at SKK’s sole cost, to confirm that the requirements set forth in Section 7.6.1 are satisfied. Such audit may be conducted only upon at least thirty (30) days prior written notice to Can-Fite, and Can-Fite agrees to accept SKK’s representatives at Can-Fite’s Ingredient manufacturing facility for such audit purpose. If SKK’s representatives that are assigned to perform such audit are not SKK’s employees, officers or directors, then any permitted access to Can-Fite’s Facility by such SKK representatives shall be subject to Can-Fite’s (and/or its Third Party manufacturer(s)’ (if applicable)) prior consent, which consent by Can-Fite shall not be unreasonably withheld or delayed. If MHLW requirements do not permit SKK to delegate Ingredient manufacturing facility audits to Can-Fite in accordance with this Section 7.6.2, the Parties shall promptly meet to discuss and determine reasonable and practicable means to enable SKK’s compliance with such requirements (through the Parties’ collaborative efforts in this regard).
| 24 |
Execution Copy
Confidential
7.6.3 Audit by MHLW. If Can-Fite is requested by agents of the MHLW to accept an audit of Can-Fite’s or its Third Party manufacturer’s Ingredient manufacturing facility (such request may be intermediated by SKK), which audit is permitted by Japanese law, Can-Fite agrees to accept such audit.
7.6.4 Japanese Laws and Regulations. SKK will assist Can-Fite to be familiarized with Japanese laws and regulations, since Can-Fite will be accredited as an “overseas manufacturer” and will be governed by those laws and regulations to the extent that Can-Fite exports a pharmaceutical product or an ingredient thereof into Japan. Can-Fite agrees to cause its Third Party manufacturer(s) to agree to take steps to register as an “overseas manufacturer” at the Japanese government with SKK’s assistance.
7.7 Option to Manufacture. Notwithstanding anything to the contrary herein, Can-Fite hereby grants SKK an option to manufacture or have a Third Party manufacture on SKK’s behalf (provided such Third Party contract manufacturer is approved in advance by Can-Fite, such approval not to be unreasonably withheld or delayed) the Ingredient solely for incorporation into the Product for development hereunder and/or for Product sale, promotion, distribution, use and other commercial purposes in the Field in the Territory.
7.7.1 Exercise of Option. SKK may exercise such option at any time during the term hereof upon giving Can-Fite one hundred twenty (120) days’ prior written notice of its intent to exercise the option and paying Can-Fite an option exercise fee of One Million U.S. Dollars ($1,000,000) within thirty (30) days after sending such notice to Can-Fite. Upon exercise of such option (i.e., upon SKK’s delivery of both the written notice and the option exercise fee), Can-Fite will grant to SKK a non-exclusive license to manufacture or have manufactured Ingredient in the Territory (and to manufacture or have manufactured Ingredient outside the Territory solely for incorporation into Product, for Product sale, promotion, distribution, use and other commercial purposes in the Field in the Territory) to meet all or a portion of the requirements of SKK and its Sublicensees for Ingredient in the Territory. Within the 120-day period after receipt of such written notice and payment, Can-Fite will use Commercially Reasonable Efforts to support the transfer of relevant Ingredient manufacturing information to SKK or its approved contract manufacturer, including transfer of the then-current manufacturing technology with respect to Ingredient, including but not limited to relevant know-how relating to the Ingredient manufacturing process, pertinent aspects of Can-Fite’s Ingredient manufacturing facility and raw material source (subject to confidentiality and use restrictions). SKK shall pay Can-Fite all costs associated with such technology and information transfer within thirty (30) days after the date of invoice(s) therefor submitted to SKK by Can-Fite.
| 25 |
Execution Copy
Confidential
7.7.2 Manufacturing Royalty. Upon the manufacture of Ingredient by or on behalf of SKK following exercise of the option hereunder, SKK shall pay to Can-Fite, in addition to all other amounts payable hereunder, including royalty payments under Section 9.5, a manufacturing royalty equal to two and one-half percent (2.5%) of the Reimbursement Price for the units of Product that contain Ingredient manufactured by or on behalf of SKK, to be paid quarterly in accordance with the applicable provisions of Sections 9.5 through 9.10.
ARTICLE 8.
SALES AND MARKETING
8.1 Marketing Efforts. SKK agrees to use its Commercially Reasonable Efforts to (i) launch commercial sales of the Product in the Territory as soon as possible after receipt of the Marketing Authorization for the Product in the Territory; and (ii) after Commercial Launch of the Product in the Territory, maximize the Net Sales in the Territory.
8.2 Marketing Plans. SKK shall prepare marketing plans for the Territory (the “Marketing Plans”), which shall include plans related to the pre-launch, launch, promotion and sale of the Product in the Territory. SKK shall share with Can-Fite the Marketing Plans on a regular basis, but no less frequently than annually. In addition, SKK shall keep Can-Fite informed, as requested by Can-Fite, with respect to the marketing, sales and promotion of the Product in the Territory. SKK shall have full control and authority over of the day-to-day commercialization of the Product in the Territory and implementation of the corresponding Marketing Plans, at SKK’s sole expense.
8.3 Marketing Materials. For purposes of harmonization and coordination of global commercialization of the Product, each Party shall keep the other Party informed regarding the preparation of promotional materials, samples, advertising and materials for training sales representatives with respect to the Product. Upon reasonable request of a Party, the other Party shall provide copies of such Product-related written materials. SKK shall have sole responsibility for the Product marketing materials used in the Territory.
ARTICLE 9.
MILESTONES, ROYALTIES AND OTHER PAYMENTS
9.1 Upfront and Annual Payments.
9.1.1 Upfront Payment. Within seven (7) business days after the Effective Date, SKK shall pay to Can-Fite the non-refundable, non-creditable amount of Three Million U.S. Dollars ($3,000,000).
9.1.2 Annual Payment. Commencing January 1, 2007 and on January 1 of each year thereafter until the earlier of (i) the filing by SKK of a New Drug Application with a Regulatory Authority in Japan for the first indication or (ii) the sixth (6th) anniversary of the Effective Date, SKK shall pay to Can-Fite the non-refundable, non-creditable amount of Five Hundred Thousand U.S. Dollars ($500,000).
| 26 |
Execution Copy
Confidential
9.2 Milestone Payments. Within thirty (30) days following the first achievement or occurrence of each of the following milestone events by performance of SKK or an Affiliate or Sublicensee of SKK, SKK shall pay to Can-Fite the corresponding one-time, non-creditable, non-refundable milestone payments set forth herein:
| Milestone Event | Milestone Payment | ||
| (i) | Upon Marketing Authorization in Japan for rheumatoid arthritis or other first indication |
Five Million U.S. Dollars ($5,000,000) | |
| (ii) | Upon Marketing Authorization in Japan for the second indication |
Three Million U.S. Dollars ($3,000,000) | |
| (iii) | Upon commencement of first Clinical Study in Japan, whether or not SKK employs Bridging Strategy |
One Million U.S. Dollars ($1,000,000) | |
| (iv) | Upon commencement of Phase II Clinical Trial in Japan for the first indication, whether or not SKK employs Bridging Strategy |
One and One-Half Million U.S. Dollars ($1,500,000) | |
| (v) | Upon submission of NDA to Regulatory Authority in Japan for first indication, whether or not SKK employs bridging strategy |
Two and One-Half Million U.S. Dollars ($2,500,000) | |
| (vi) | If SKK does not employ Bridging Strategy: upon commencement of Phase III Clinical Trial in Japan for first indication |
Two Million U.S. Dollars ($2,000,000) | |
| (vii) | Commencement of each Phase III Clinical Trial in Japan for each indication after first indication |
One Million U.S. Dollars ($1,000,000) | |
For the avoidance of doubt, each milestone payment will be nonrefundable and noncreditable against royalties payable pursuant to Section 9.5 and any other fees or other payments due Can-Fite under this Agreement or under the Supply Agreement.
9.3 Participation in Development Costs. In addition to all milestone payments and royalties hereunder, SKK shall pay Can-Fite the following:
9.3.1 Development Milestones. SKK shall pay to Can-Fite Two Million U.S. Dollars ($2,000,000) toward the costs of Can-Fite’s Phase IIb Clinical Trial of the Ingredient for rheumatoid arthritis (Protocol Number CF101-202RA) in accordance with the following schedule: (i) Five Hundred Thousand U.S. Dollars ($500,000) upon commencement of such Phase IIb Clinical Trial; (ii) Five Hundred Thousand U.S. Dollars ($500,000) upon enrollment of fifty percent (50%) of the patients or subjects to be enrolled in such Phase IIb Clinical Trial; (iii) Five Hundred Thousand U.S. Dollars ($500,000 ) upon enrollment of one hundred percent (100%) of the patients or subjects to be enrolled in such Phase IIb Clinical Trial; and (iv) Five Hundred Thousand U.S. Dollars ($500,000) upon SKK’s receipt of a copy of the final report of such Phase IIb Clinical Trial. Can-Fite shall notify SKK in writing upon the occurrence of each of the foregoing payment trigger events and SKK shall pay Can-Fite within thirty (30) days of such notice.
| 27 |
Execution Copy
Confidential
9.3.2 Phase III Clinical Trial Full Reports and Cost-Sharing. In accordance with Section 4.3, if SKK requests a copy of the full report of any Phase III Clinical Trial performed by Can-Fite for the purpose of Can-Fite’s filing a New Drug Application in the United States for marketing the Product for rheumatoid arthritis, Can-Fite shall forward to SKK the a copy of the full report of such Phase III Clinical Study. If the Japanese Regulatory Authority accepts the Bridging Strategy and SKK decides to employ the Bridging Strategy, SKK shall so notify Can-Fite in writing and shall reimburse Can-Fite for thirty percent (30%) of the Clinical Study Costs of Can-Fite’s Phase III Clinical Trial performed by Can-Fite for the purpose of Can-Fite’s filing a New Drug Application in the United States for marketing the Product for rheumatoid arthritis. SKK shall make such payment within thirty (30) days after Can-Fite (or its Affiliate, Can-Fite’s Other Licensee or agent, on behalf of Can-Fite) delivers an invoice therefor to SKK, which invoice shall set forth in reasonable detail various categories and amounts within such Clinical Study Costs (provided that such categories will be consistent with Can-Fite’s standard internal accounting procedures).
9.3.3 Clinical Study Full Reports and Cost-Sharing. For any Clinical Study commenced by or on behalf of Can-Fite after the Effective Date for which SKK does not pay a portion of costs pursuant to Section 9.3.1 or 9.3.2, if SKK requests a copy of the full report of such Clinical Study in accordance with Section 4.3, Can-Fite shall forward to SKK a copy of the full report of such Clinical Study. SKK shall reimburse Can-Fite for twenty-five percent (25%) of the Clinical Study Costs incurred in connection with each such Clinical Study for which SKK has requested a copy of the corresponding full report. SKK shall make such payment within thirty (30) days after Can-Fite (or its Affiliate, Can-Fite’s Other Licensee or agent, on behalf of Can-Fite) delivers an invoice therefor to SKK, which invoice shall set forth in reasonable detail various categories and amounts within such Clinical Study Costs (provided that such categories will be consistent with Can-Fite’s standard internal accounting procedures).
9.3.4 Non-Clinical Study Full Reports and Cost-Sharing. For any Non-Clinical Studies commenced by or on behalf of Can-Fite after the Effective Date, if SKK requests a copy of the full report of a given Non-Clinical Study in accordance with Section 4.3, Can-Fite shall forward to SKK a copy of the full report of such Non-Clinical Study. SKK shall reimburse Can-Fite for twenty percent (20%) of Can-Fite’s Non-Clinical Study Costs incurred in connection with each such Non-Clinical Study for which SKK has requested a copy of the corresponding full report (wherein such Non-Clinical Study Costs shall be determined in a manner that is analogous to determination of Clinical Study Costs hereunder). SKK shall make such payment within thirty (30) days after Can-Fite (or its Affiliate, Can-Fite’s Other Licensee or agent, on behalf of Can-Fite) delivers an invoice therefor to SKK, which invoice shall set forth in reasonable detail various categories and amounts within such Non-Clinical Study Costs (provided that such categories will be consistent with Can-Fite’s standard internal accounting procedures).
| 28 |
Execution Copy
Confidential
9.4 SKK’s Data and Cost-Sharing. If, in accordance with Section 4.3, Can-Fite requests a copy of the full report of a given Clinical Study or a given Non-Clinical Study performed by or on behalf of SKK, SKK shall forward to Can-Fite a copy of the full report of such requested Clinical Study or Non-Clinical Study, as the case may be. Can-Fite shall reimburse SKK for eighty percent (80%) of SKK’s Non-Clinical Study Costs incurred in connection with such Non-Clinical Study for which Can-Fite has requested a copy of the corresponding full report (wherein such Non-Clinical Study Costs shall be determined in a manner that is analogous to determination of Clinical Study Costs hereunder), and Can-Fite shall reimburse SKK for seventy-five percent (75%) of SKK’s Clinical Study Costs incurred in connection with such Clinical Study for which Can-Fite has requested a copy of the corresponding full report. Can-Fite shall make such payment within thirty (30) days after SKK (or its Affiliate, Sublicensee or agent, on behalf of SKK) delivers an invoice therefor to Can-Fite, which invoice shall set forth in reasonable detail various categories and amounts within such Non-Clinical Study Costs or Clinical Study Costs, as the case may be (provided that such categories will be consistent with SKK’s standard internal accounting procedures).
9.5 Royalties.
9.5.1 Royalty Rates. Subject to Section 9.5.2, SKK shall pay to Can-Fite a royalty, based on the following royalty rates, for annual Net Sales in the Territory: (i) seven percent (7%) of that portion of annual Net Sales in the Territory that is less than or equal to Seventy Million U.S. Dollars ($70,000,000) and (ii) twelve percent (12%) of that portion of annual Net Sales in the Territory that is greater than Seventy Million U.S. Dollars ($70,000,000).
9.5.2 Can-Fite’s Right to Receive Section 9.5.1 Royalties; Reduced Royalty Rate. Can-Fite’s right to receive royalties at the rates set forth in Section 9.5.1 will be in effect until the later of: (i) the first six (6) year period during which a generic product incorporating the Ingredient is prevented by law, rules or regulations in the Territory from being launched in the Territory, or (ii) the date of expiration of the last-to-expire of the Licensed Patents containing a Valid Claim that, but for the license granted by Can-Fite to SKK hereunder, would be directly or contributorily infringed by the use or sale of the Product in the Territory. After such time and until ten (10) years after the date of Commercial Launch of Product in the Territory, SKK shall pay to Can-Fite a royalty based on a royalty rate of four percent (4%) of annual Net Sales.
| 29 |
Execution Copy
Confidential
9.5.3 Paid-Up License. Upon expiration of this Agreement, and SKK’s payment in full of the royalty amounts due and owing under this Section 9.5, SKK shall acquire a fully paid-up license under the Licensed Technology and Data to continue commercialization activities relating to the Product, without making any further payment to Can-Fite. SKK is entitled to extend such fully paid-up license to its Sublicensees.
9.5.4 Timing of Royalty Payments. All royalties payable to Can-Fite under this Agreement will be paid by SKK within sixty (60) days of the end of each calendar quarter.
9.6 Payment Method; Currency Conversion. All payments under this Agreement shall be made by wire transfer or other means acceptable to Can-Fite, as specified by Can-Fite. All dollar amounts specified in this Agreement, and all payments made hereunder, are and shall be made in U.S. dollars. Royalties, and any other payments due under this Agreement that are calculated based on amounts received by SKK or its Affiliates or Sublicensees in currencies other than U.S. dollars will be converted into the U.S. dollar equivalent using the applicable conversion rate as reported in the Exchange Rates set forth in Japanese version of The Wall Street Journal for the last Business Day of the calendar quarter to which such payments relate.
9.7 Late Payments. Any payments due under this Agreement that are not paid by the date such payments are due shall bear interest at the lesser of: (i) the average one-month London Interbank Offering Rate for the United States Dollar as reported from time to time in The Wall Street Journal, effective for the first date on which payment was delinquent and calculated on the number of days such payment is overdue or, if such rate is not regularly published, as published in such source as the Parties agree plus three (3) percentage points per annum, or (ii) the maximum amount permitted by law, calculated from the date payment was initially due. The foregoing interest shall be due from SKK without any special notice and shall be in addition to any other remedies that Can-Fite may have pursuant to this Agreement.
9.8 Withholding Tax. If any payment due to Can-Fite hereunder is subject to withholding taxes or similar governmental charge (“Withholding Tax”) required to be paid or withheld thereon by applicable law in Japan and such Withholding Tax is creditable against income taxes required to be paid in Israel by Can-Fite in its nature, then SKK shall deduct such Withholding Tax from such payment due Can-Fite hereunder at a rate not to exceed the then-prevailing rate provided for in applicable provisions of the Conventions between the Governments of Israel and Japan for the Avoidance of Double Taxation and the Evasion of Taxes dated March 3, 1993 (effective January 1, 1994). SKK shall provide Can-Fite, as soon as possible, a certificate evidencing withholding or payment of any such Withholding Tax by SKK, its Affiliates or its Sublicensees for the benefit of Can-Fite. Any other duty, tax, charge levied thereon outside Israel shall be borne and paid by SKK without deduction from such payment due Can-Fite.
| 30 |
Execution Copy
Confidential
9.9 Reports and Records. During the term of this Agreement, SKK shall furnish to Can-Fite a written quarterly report showing: (i) the amount of gross sales of Product by SKK, its Affiliates, its distributors and Sublicensees to wholesalers and other Third-Party purchasers, and an itemized calculation of Net Sales of each Product during such calendar quarter by SKK, its Affiliates, its distributors and Sublicensees, (ii) the amounts payable in United States dollars which shall have accrued in respect of such Net Sales and the calculation thereof; (iii) Withholding Tax, if any; and (iv) the exchange rates used in determining the conversion to and amount of United States dollars. The foregoing quarterly report shall be certified by an executive officer of SKK as consistent with SKK’s standard practices in performing such computations and in accordance with SKK’s standard internal accounting procedures. SKK will keep or cause to be kept such records as are required in sufficient detail to track and determine (in accordance with SKK’s standard internal accounting procedures) the accuracy of calculations of all sums due under this Agreement and to accurately account for the calculations of all royalties due under this Agreement. Such records will be retained for a period of the longer of (xi) a three (3) year period following the year in which any payments were made hereunder and (xii) the expiration of the applicable tax statute of limitations (or any extensions thereof), or such longer period as may be required by law.
9.10 Records; Audit by Can-Fite. Once per calendar year and within three (3) years from Can-Fite’s receipt of each royalty payment, and for each Clinical Study or Non-Clinical Study for which Can-Fite reimburses SKK a portion of Clinical Study Costs or Non-Clinical Study Costs pursuant to Section 9.4, within three (3) years from the completion of such Clinical Study or Non-Clinical Study (as applicable), Can-Fite will have the option to engage (at its own expense) an independent certified public accountant, appointed by Can-Fite and reasonably acceptable to SKK, to examine in confidence the books and records of SKK as may be necessary to determine, with respect to any calendar year, the correctness or completeness of any report or payment required to be made under this Agreement; provided however, that the books and records for any particular calendar year will only be subject to one audit. The report of such accountant will be limited to a certificate verifying any report made or payment submitted by SKK during such period or identifying any over-payment or under-payment made by SKK, and/or any amount of Clinical Study Costs or Non-Clinical Study Costs (as applicable), accompanied by an explanation of the basis for its determination of such over-payment or under-payment and/or such over-charging or under-charging. In addition, if the accountant is unable to verify the correctness of any such payment, the accountant’s report may include information relating to why such payment is unverifiable. If the audit reveals any underpayment by SKK to Can-Fite or any over-charging of Clinical Study Costs or Non-Clinical Study Costs (as applicable) reimbursed by Can-Fite to SKK, then SKK will pay any underpayment to Can-Fite and/or refund any overcharged amount to Can-Fite, together with all interest accrued thereon, within thirty (30) days after SKK’s receipt of the audit report. If any audit performed under this Section 9.10 discloses a deficiency of more than five percent (5%) from the amount of the original report showing the calculation of a royalty under Section 9.5 and/or an overpayment of Clinical Study Costs or Non-Clinical Study Costs (as applicable) by Can-Fite of more than five percent (5%) from the amount of the original report showing the calculation of an amount payable under Section 9.4, SKK will bear the full cost of the performance of such audit. The result of the audit and the audit report shall be subject to Article 13.
| 31 |
Execution Copy
Confidential
9.11 Audit by SKK. For each Clinical Study and Non-Clinical Study for which SKK reimburses Can-Fite a portion of Clinical Study Costs or Non-Clinical Study Costs pursuant to Section 9.3.2, 9.3.3 or 9.3.4, and within three (3) years from the completion of such Clinical Study or Non-Clinical Study (as applicable), SKK will have the option to engage (at its own expense) an independent certified public accountant, appointed by SKK and reasonably acceptable to Can-Fite, to examine in confidence the books and records of Can-Fite as may be necessary to determine the correctness or completeness of any amount of Clinical Study Costs or Non-Clinical Study Costs. The report of such accountant will be limited to a certificate verifying any amount of Clinical Study Costs or Non-Clinical Study Costs, accompanied by an explanation of the basis for its determination of such over-charging or under-charging. In addition, if the accountant is unable to verify the correctness of any such payment, the accountant’s report may include information relating to why such payment is unverifiable. If the audit reveals any over charging of Clinical Study Costs or Non-Clinical Study Costs reimbursed by SKK to Can-Fite, then Can-Fite will refund any over-charged amount to SKK, together with all interest accrued thereon, within thirty (30) days after Can-Fite’s receipt of the audit report. If any audit performed under this Section 9.11 discloses an overpayment of Clinical Study Costs or Non-Clinical Study Costs by SKK of more than five percent (5%) from the amount of the original report showing the calculation of an amount payable under Section 9.3.2, 9.3.3 or 9.3.4, Can-Fite will bear the full cost of the performance of such audit. The result of the audit and the audit report shall be subject to Article 13.
ARTICLE 10.
INTELLECTUAL PROPERTY
10.1 Prosecution and Maintenance. Can-Fite shall own or Control (as applicable), be responsible for, and shall diligently carry out and shall bear all costs (including attorneys’ fees) for the preparation, filing, prosecution, maintenance, and extensions, if any, of all patents or patent applications within the Licensed Patents in the Territory. Can-Fite shall have the right, after consultation with SKK, and upon no less than thirty (30) days’ notice, to abandon any of the Licensed Patents in the Territory. After good faith consideration of Can-Fite’s reasons for such abandonment of a patent and/or patent application within the Licensed Patents in the Territory, as well as due consideration of any actual or potential adverse effects on Licensed Patents within or outside of the Territory that would or may result from continuation of such patent or patent application, SKK shall have the right to direct Can-Fite to continue the prosecution or maintenance of any patent or patent application that Can-Fite wishes to abandon, in Can-Fite’s name and at SKK’s sole cost and expense. For the avoidance of doubt, Can-Fite may take ministerial and non-material procedural actions regarding the Licensed Patents in the Territory without obtaining prior input from SKK.
10.2 Inventions.
10.2.1 Inventorship. Inventorship of information, know-how, data, discoveries, developments, designs, inventions, methods, processes, techniques, materials, formulae, trade secrets, trademarks, copyrights, patents and patent applications and other proprietary information conceived and/or reduced to practice in connection with, or as a result of, SKK’s activities hereunder and that are related to Ingredient and/or Product (“Inventions”) shall be determined in accordance with the patent laws of the country in which such invention occurred.
| 32 |
Execution Copy
Confidential
10.2.2 Ownership of Inventions; Royalty-Free Licenses; Responsibility for Patent Procurement. If an Invention is made solely by employees, officers, directors, agents or consultants of SKK, and such Invention specifically relates to development of the Product by or on behalf of SKK, the ownership of such Invention shall be vested solely in SKK (each an “SKK Invention”). SKK hereby grants to Can-Fite a royalty-free, non-exclusive license to use and exploit SKK Inventions in connection with the Ingredient and Product outside of the Territory. All other Inventions (whether invented solely by Can-Fite or jointly by Can-Fite and SKK) shall belong to Can-Fite (each a “Can-Fite Invention”). Can-Fite hereby grants to SKK a royalty-free, non-exclusive license to use and exploit Can-Fite Inventions in connection with the Ingredient and Product in accordance with this Agreement. SKK shall prepare, file, prosecute and maintain any and all patents and patent applications related to SKK Inventions; Can-Fite shall prepare, file, prosecute and maintain any and all patents and patent applications related to Can-Fite Inventions.
10.3 Enforcement of Licensed Technology. If either Can-Fite or SKK has knowledge of any infringement or likely infringement of the Licensed Patents or unauthorized use of the Licensed Know-How in the Territory, then the Party having such knowledge shall promptly inform the other Party in writing, and the Parties shall promptly consult with one another regarding the action to be taken. Unless the Parties otherwise mutually agree, Can-Fite shall have the initial right, using counsel of its choice, to enforce such Licensed Technology or defend any declaratory action with respect thereto, at its sole expense, and SKK shall give all reasonable assistance to Can-Fite in such action. If Can-Fite exercises such right, then Can-Fite shall control the strategy of such action and, provided that Can-Fite either receives SKK’s consent or is required by law, Can-Fite may use SKK’s name in connection with such action. If the infringement or likely infringement of the Licensed Patents would be the basis of a potential action solely within the Field in the Territory, and if Can-Fite declines to commence such action, then SKK shall have the right, but not the obligation, to commence such declined action with respect to such infringement within the Field in the Territory; provided that, prior to SKK’s commencement of any such declined action, SKK shall reasonably consider Can-Fite’s reasons for declining to commence the action. In the event that SKK elects, in its sole discretion and at SKK’s sole expense, to commence such declined action, (i) SKK shall reasonably consider Can-Fite’s input with respect to such declined action; (ii) Can-Fite shall give all reasonable assistance to SKK in such action; and (iii) SKK may use Can-Fite’s name in connection with such action. SKK shall keep Can-Fite reasonably apprised of the progress of any such action commenced by SKK.
10.4 Infringement of Third Party Patents. If SKK, or any of its Affiliates or Sublicensees, is sued by a Third Party for infringement of a Third Party’s patent rights in the Territory because of the manufacture, use or sale of the Product in the Territory, SKK shall promptly notify Can-Fite in writing of such suit, and the Parties shall consult each other to agree upon the course of action to be taken. Unless otherwise agreed in writing by the Parties, Can-Fite shall have the first right, but not the obligation, to control the defense of such suit in the Territory with counsel of its choice, at its own expense, in which event SKK shall have the right to be represented by advisory counsel of its own selection at its own expense, and SKK shall reasonably cooperate in the defense of such suit and furnish to Can-Fite all pertinent evidence and reasonable assistance in SKK’s control. The Party that is not controlling the defense of such suit shall cooperate with the Party that is controlling the defense of such suit in connection with any such claim, suit or proceeding, and each Party shall keep the other Party reasonably informed of all material developments in connection with any such claim, suit or proceeding.
| 33 |
Execution Copy
Confidential
10.5 Recoveries; Settlement. In the event that either Party recovers any amounts from any litigation or settlement under Section 10.3 or 10.4, such amounts shall first be applied to reimburse Can-Fite and SKK for their respective actual out-of-pocket expenses, or equitable proportions thereof. Any remaining amount shall be retained by the Party that controlled such litigation or entered into such settlement; provided, however, that if SKK is the Party retaining any such remaining amount, then such remaining amount shall be deemed to be Product sales hereunder, and shall be subject to the royalty payments set forth in Section 9.5. The Parties shall keep one another informed of their respective activities concerning, and the status of, any litigation or settlement thereof concerning an Invention, the Licensed Technology, the Ingredient or the Product; provided, however, that no settlement or consent judgment or other voluntary final disposition of any suit defended or action brought by a Party pursuant to this Article 10 may be entered into without the written consent of the other Party if such settlement would require the other Party to be subject to an injunction or to make a monetary payment or would otherwise adversely affect the other Party’s rights under this Agreement.
10.6 Trademark Infringement. SKK shall promptly call to the attention of Can-Fite the use by any Third Party of any Trademark or any trademark similar to the Trademarks, of which it becomes aware. Can-Fite shall have the right to decide whether or not to bring proceedings against such Third Parties, giving commercially reasonable consideration to any reasonably anticipated, material adverse effect(s) on SKK’s business (to the extent SKK has provided written information to Can-Fite regarding such reasonably anticipated, material adverse effect(s)). Such proceedings shall be at the expense of Can-Fite. SKK shall cooperate fully with Can-Fite to whatever extent is deemed reasonably necessary by Can-Fite to prosecute such action. In the event that Can-Fite recovers damages from prosecution of such action, Can-Fite shall retain all amounts received for such damages, except that SKK shall be entitled to reimbursement of its costs, expenses, and attorneys’ fees attributable to such action (or in proportionate amounts thereof, should Can-Fite recover an insufficient amount for both Parties’ such costs and expenses).
| 34 |
Execution Copy
Confidential
ARTICLE
11.
REPRESENTATIONS AND WARRANTIES; LIMITATION OF LIABILITY
11.1 Can-Fite Representations and Warranties. Can-Fite hereby represents and warrants as of the Effective Date that: (i) it has the right, power and corporate authority to enter into this Agreement and to make the promises set forth in this Agreement; (ii) it owns or Controls the Licensed Patents and has the right to grant the rights and licenses herein to SKK in the Territory; (iii) the execution, delivery and performance of this Agreement do not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which it is bound, nor to its Knowledge, violate any law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it; (iv) there are no actual or, to its Knowledge, threatened suits or claims by any Third Party alleging that the use by Can-Fite or SKK of the Licensed Technology will constitute an infringement or other violation of a patent of such Third Party; and (v) Can-Fite has disclosed to SKK, in writing or in electronic form, (a) material (individually or in the aggregate) information in connection with the List of Can-Fite Studies; (b) detailed information under Can-Fite’s control relating to the Ingredient manufacturing process used by Can-Fite as of the Effective Date (“Manufacturing Process”), wherein such Manufacturing Process information was provided to SKK before the Effective Date; and (c) pertinent information under Can-Fite’s control provided to SKK before the Effective Date relating to the use of the Product obtained from Clinical Studies and Non-Clinical Studies performed by Can-Fite.
11.2 SKK Representations and Warranties. SKK hereby represents and warrants as of the Effective Date that: (i) it has the right, power and corporate authority to enter into this Agreement and to make the promises set forth in this Agreement; and (ii) the execution, delivery and performance of this Agreement do not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which it is bound, nor to its Knowledge, violate any law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it.
11.3 Disclaimer of Warranties. EXCEPT AS OTHERWISE EXPRESSLY STATED IN THIS AGREEMENT, CAN-FITE EXPRESSLY DISCLAIMS ANY WARRANTIES, REPRESENTATIONS OR CONDITIONS, EXPRESS, IMPLIED, STATUTORY OR OTHERWISE, WITH RESPECT TO THE CONFIDENTIAL INFORMATION, INGREDIENT, PRODUCT, MANUFACTURING PROCESS, LICENSED PATENTS OR LICENSED KNOW-HOW, INCLUDING, WITHOUT LIMITATION, ANY WARRANTY OF MERCHANTABILITY, NONINFRINGEMENT, OR FITNESS FOR A PARTICULAR PURPOSE, VALIDITY OF THE LICENSED PATENTS.
11.4 Limitation of Liability. NEITHER PARTY SHALL BE LIABLE TO THE OTHER PARTY FOR ANY SPECIAL, CONSEQUENTIAL, INDIRECT, OR INCIDENTAL DAMAGES OF ANY KIND (INCLUDING DAMAGES FOR INTERRUPTION OF BUSINESS, PROCUREMENT OF SUBSTITUTE GOODS, LOSS OF PROFITS, OR THE LIKE) ARISING OUT OF OR RELATING TO THIS AGREEMENT, REGARDLESS OF WHETHER SUCH DAMAGES ARE BASED ON TORT, WARRANTY, CONTRACT OR ANY OTHER LEGAL THEORY, EVEN IF ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. THIS SECTION SHALL BE GIVEN FULL EFFECT EVEN IF ANY REMEDY SPECIFIED IN THIS AGREEMENT IS DEEMED TO HAVE FAILED OF ITS ESSENTIAL PURPOSE.
| 35 |
Execution Copy
Confidential
ARTICLE 12.
INDEMNIFICATION AND INSURANCE
12.1 By Can-Fite. Can-Fite shall indemnify, defend and hold SKK, its Affiliates, directors, Sublicensees, employees, agents and representatives (collectively, “SKK Indemnitees”) harmless from and against all claims, causes of action, costs (including reasonable attorney fees and expenses), losses or liabilities (collectively, “Losses”) of any kind that are asserted by a Third Party to the extent the Losses arise from: (i) breach of a representation or warranty by Can-Fite in Section 11.1; (ii) the negligent act or omission or willful misconduct of Can-Fite in the performance of its obligations under this Agreement; or (iii) manufacture of Ingredient produced by Can-Fite or Can-Fite’s Third Party manufacturer not in compliance with the Specifications, or not in compliance with the Manufacturing Process, or Losses that directly result from Can-Fite’s failure to inform SKK of any material change to the Manufacturing Process thirty (30) days prior to implementation of such material change. The foregoing indemnity under subsections (i) – (iii) shall not apply to the extent that any of the SKK Indemnitees caused or contributed to such Losses, or to the extent that SKK has an indemnification obligation under Section 12.2 with respect to the Losses.
12.2 By SKK. SKK shall indemnify, defend and hold Can-Fite, its Affiliates, Can-Fite Other Licensee(s), directors, employees, agents and representatives (collectively, “Can-Fite Indemnitees”) harmless from and against all Losses of any kind that are asserted by a Third Party to the extent the Losses arise from: (i) breach of a representation or warranty by SKK in Section 11.2; (ii) the negligent act or omission or willful misconduct of SKK or any of its Affiliates, Sublicensees, agents or representatives in the performance of their obligations under this Agreement; or (iii) the development, manufacture, marketing, selling, handling or distribution by or on behalf of SKK of the Ingredient or Product (as applicable) in the Territory. The foregoing indemnity under subsections (i) – (iii) shall not apply to the extent that any of the Can-Fite Indemnitees caused or contributed to such Losses, or to the extent that Can-Fite has an indemnification obligation under Section 12.1 with respect to the Losses.
12.3 Procedure. Each Party will promptly notify the other Party in writing in the event it becomes aware of a Third Party claim, action or suit for which indemnification may be sought hereunder (provided that the failure to give such notice promptly will not prejudice the rights of an Indemnified Party, except to the extent that the failure to give such prompt notice materially adversely affects the ability of the Indemnifying Party to defend the claim, action or suit). In the event that any Third Party claim, action or suit is instituted against a Party in respect of which indemnity may be sought pursuant to this Article 12, promptly after such Party (the “Indemnified Party”) notifies the other Party (the “Indemnifying Party”) in writing, the Indemnifying Party and the Indemnified Party shall meet to discuss how to respond to such claim, action or suit. The Indemnifying Party shall control the defense of such claim, action or suit. The Indemnified Party shall cooperate with the Indemnifying Party in the defense of such claim, action or suit, at the expense of the Indemnifying Party. In any such proceeding, the Indemnified Party shall also have the right to retain its own counsel at its own expense. The Indemnifying Party shall not be liable for Losses or Third Party liabilities with respect to a claim, action or suit settled or compromised by the Indemnified Party without the Indemnifying Party’s prior written consent. No offer of settlement, settlement or compromise by the Indemnifying Party shall be binding on an Indemnified Party without the Indemnified Party’s prior written consent (which consent shall not be unreasonably withheld or delayed), unless such settlement fully releases the Indemnified Party without any liability, loss, cost or obligation to such Indemnified Party.
| 36 |
Execution Copy
Confidential
12.4 Insurance. SKK and Can-Fite each, at its own cost, shall maintain comprehensive general liability (“CGL”) insurance, including broad form contractual liability and product liability coverages, in amounts customary in the pharmaceutical industry. Each Party shall maintain such insurance during the term of this Agreement and thereafter for a period of two (2) years. Each Party, upon request, shall provide the other Party with a certificate of insurance as evidence of such coverages, and shall give the other Party at least thirty (30) days notice of any cancellation, termination or change in such insurance.
ARTICLE
13.
CONFIDENTIALITY AND PUBLICITY
13.1 Treatment of Confidential Information. The Parties agree that during the term of this Agreement, and for a period of five (5) years after this Agreement expires or terminates, the Receiving Party of Confidential Information of the Disclosing Party will (i) maintain such Confidential Information in confidence to the same extent the Receiving Party maintains its own confidential or proprietary information or trade secrets of similar kind and value; (ii) not disclose such Confidential Information to any Third Party without the prior written consent of the Disclosing Party, except for disclosures to its Affiliates, Sublicensees and Can-Fite Other Licensee(s) who agree to be bound by obligations of non-disclosure and non-use at least as stringent as those contained in this Article 13; and (iii) not use Confidential Information for any purpose except those purposes permitted by this Agreement. Neither Party will knowingly disclose to the other Party any Third Party information or know-how that such Party does not have the legal right to disclose to the other Party and/or which it has a contractual obligation not to disclose to the other Party.
13.2 Authorized Disclosure. Notwithstanding the foregoing Section 13.1, a Receiving Party may disclose Confidential Information of the Disclosing Party:
| (i) | to the extent and to the persons and entities as required by an applicable law, rule, regulation, legal process, court order or the rules of the any securities exchange on which any security issued by either Party is traded or of a Regulatory Authority; or |
| (ii) | as necessary to file, prosecute or defend those patent applications or patents for which either Party has the right to assume filing, prosecution, defense or maintenance, pursuant to Article 10 of this Agreement; or |
| (iii) | to prosecute or defend litigation or otherwise establish rights or enforce obligations under this Agreement, but only to the extent that any disclosure is necessary. |
| 37 |
Execution Copy
Confidential
Provided that, the Receiving Party required or intending to disclose the Disclosing Party’s Confidential Information under Sections 13.2(i) or (iii) shall give advance written notice to the Disclosing Party of such required disclosure so that the Disclosing Party may seek a protective order or other appropriate remedy. If, in the absence of a protective order or other remedy, the Receiving Party is nonetheless, in the reasonable opinion of Receiving Party’s counsel, required to disclose Confidential Information of the Disclosing Party under Sections 13.2(i) or (iii), the Receiving Party may disclose only that portion of the Confidential Information of the Disclosing Party which such counsel advises in writing is legally required to be disclosed; provided that the Receiving Party shall preserve the confidentiality of such Confidential Information to the fullest extent possible, including, without limitation, by cooperating with the Disclosing Party in its efforts to secure confidential or protective treatment of such Confidential Information or to obtain a protective order or other remedy.
13.3 Other Permitted Disclosures. Either Party may disclose Confidential Information received under this Agreement to existing or potential investors, acquirers, merger partners, collaborators, consultants, contractors, distributors or licensees, or to professional advisors (e.g., attorneys, accountants and investment bankers) involved in such activities, for the limited purpose of evaluating such investment, transaction, or license and under appropriate conditions of confidentiality, only to the extent necessary and with the agreement by these permitted individuals to maintain such Confidential Information in strict confidence.
13.4 Publicity; Terms of this Agreement. The Parties will mutually agree upon the text of a press release announcing the execution of this Agreement. Except for such press release, neither Party shall (i) originate any publicity, news release or other public announcement, written or oral, whether to the public press, stockholders or otherwise, relating to this Agreement, any amendment hereto or performance hereunder, or (ii) use the name of the other Party in any publicity, news release or other public announcement, except (a) with the prior written consent of the other Party, which consent shall not be unreasonably withheld or delayed, or (b) as required by applicable law, in which case the originating Party shall submit to the other Party (for review and any proposed modifications, as well as the Parties’ coordination, prior to such disclosure or use) each such required disclosure, and shall comply with the terms of Section 13.2. The terms of this Agreement shall be deemed to be the Confidential Information of each Party.
ARTICLE 14.
TERM AND TERMINATION
14.1 Term of this Agreement. This Agreement will become effective on the Effective Date and, unless earlier terminated pursuant to this Article 14, will remain in full force and effect until there is no remaining royalty payment obligation in the Territory, as set forth in Section 9.5.2. The terms and conditions for any transactions between the Parties relating to the Product after any termination or expiration hereunder shall be as separately negotiated and agreed upon by the Parties.
| 38 |
Execution Copy
Confidential
14.2 Termination for Material Breach. If either Party (the “Breaching Party”) materially breaches any of its representations, warranties, covenants or obligations under this Agreement, the other Party (the “Non-Breaching Party”) shall have the right to terminate this Agreement upon providing written notice to the Breaching Party (i) thirty (30) days after such written notice, if the Breaching Party is in breach of Article 9, 10 or 13 and has failed to cure such breach within the thirty-day notice period, or (ii) sixty (60) days after such written notice, if the Breaching Party is in breach of any other provision hereof and has failed to cure such breach within the sixty-day notice period; provided, however, that if a breach other than of Article 9, 10 or 13 is not reasonably susceptible of cure within the sixty-day cure period above, and the Breaching Party proposes and has initiated a reasonable course of action to cure such breach and has acted diligently and in good faith to begin to cure the breach within such sixty-day period, such cure period shall be extended as reasonably necessary to permit the breach to be cured. Notwithstanding the foregoing, in the event the Breaching Party disputes in good faith the existence of a breach under this Agreement, the Non-Breaching Party shall not have the right to terminate this Agreement unless and until the dispute is resolved in the Non-Breaching Party’s favor (i.e., upon a final determination that the Breaching Party has materially breached this Agreement and has failed to cure such breach) through the dispute resolution provisions of Article 15. All amounts due hereunder that are not in dispute shall continue to be timely paid.
14.3 Termination for Insolvency. This Agreement may be terminated at any time by a Party’s thirty (30) days prior written notice upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings by or against the other Party (the “Bankrupt Party”), or upon an assignment of a substantial portion of the Bankrupt Party’s assets for the benefit of its creditors; provided, however, that in the event of any involuntary bankruptcy or receivership proceeding, such right to terminate shall only become effective if the proceeding is not dismissed within sixty (60) days after the filing thereof.
14.4 Effect of Expiration or Termination.
14.4.1 Accrued Obligations. Termination of this Agreement for any reason shall not release any Party hereto from any liability which, at the time of such termination, has already accrued to the other Party or which is attributable to a period prior to such termination, nor preclude either Party from pursuing all rights and remedies it may have hereunder or at law or in equity with respect to any breach of this Agreement.
14.4.2 Survival. The expiration or termination of this Agreement shall not affect (i) the rights or obligations of either Party hereto which shall have accrued hereunder prior to such expiration or termination, and (ii) the rights and obligations of the Parties at law or in equity, which from the context thereof, are intended to survive termination or expiration of this Agreement. Without limiting the foregoing sentence, the provisions of Article 1, to the extent definitions are embodied in the following listed Articles and Sections of this Agreement; the provisions of Sections 2.1, 2.2, 2.3, 5.6, 5.7, 5.8, 5.9, 5.10, 5.11, 5.12, 8.2, 8.3 and Article 6, but only if SKK has a fully paid-up license under Section 2.1; Sections 2.6 and 2.7; Sections 7.5, 9.1, 9.2, 9.3, 9.4 and 9.5, to the extent payment obligations thereunder have accrued but not been paid; Sections 9.6, 9.7, 9.8, 9.9, 9.10, 9.11, 10.1, 10.2, 10.6, 11.3, 11.4, 14.4, 14.5, 16.3, 16.4, 16.5, 16.6, 16.7, 16.8, 16.9; Articles 12 and 13; and Article 15, with respect to Disputes arising during the term of the Agreement that have not been resolved, shall survive the expiration or termination of this Agreement for any reason. In addition, any other provision required to interpret and enforce the Parties’ rights and obligations under this Agreement shall survive, but only to the extent required for the observation and performance of the aforementioned surviving portions of this Agreement.
| 39 |
Execution Copy
Confidential
14.4.3 Termination of Licenses. Upon earlier termination of this Agreement by Can-Fite for SKK’s uncured material breach under Section 14.2 or SKK’s insolvency under Section 14.3, or by Can-Fite for SKK’s failure to proceed with Product development pursuant to Section 5.5, all licenses and rights granted to SKK hereunder shall terminate and SKK will immediately cease to develop and commercialize Product.
14.4.4 Disposition of Inventory. Upon earlier termination of this Agreement by Can-Fite for SKK’s uncured material breach under Section 14.2 or SKK’s insolvency under Section 14.3, or by Can-Fite for SKK’s failure to proceed with Product development pursuant to Section 5.5, SKK shall make no further sales of Product, and shall return to Can-Fite all of its inventory of the Product on hand as of the effective date of termination. Thereafter, Can-Fite may fill any orders for Product accepted by or on behalf of SKK prior to the effective date of termination.
14.4.5 Reassignment of Regulatory Approvals. If this Agreement is early terminated by Can-Fite under Section 14.2 because of SKK’s uncured material breach or under Section 14.3 because of SKK’s insolvency, or by Can-Fite for SKK’s failure to proceed with Product development pursuant to Section 5.5, SKK shall ensure that all Regulatory Filings and Marketing Authorizations in the Territory relating to the Product are assigned to Can-Fite (to the extent legally permissible in the Territory) within a reasonable time after termination of SKK’s rights under this Agreement, subject to Can-Fite’s payment to SKK of a two percent (2%) royalty on Net Sales of any Product that is the subject matter of such assigned Regulatory Filings and/or Marketing Authorizations; provided that such royalty payment obligation of Can-Fite shall only continue until such time that the total royalty payments delivered by Can-Fite equal an amount that reimburses SKK for all of its Non-Clinical Study Costs and Clinical Study Costs and other internal and external costs directly arising from or in connection with preparation and submission of such assigned Regulatory Filings and/or Marketing Authorizations that were reasonably borne by SKK prior to such early termination of this Agreement. Any costs incurred by SKK for such assignment or transfer shall be at SKK’s expense. In the event that no such assignment and/or transfer pursuant to this Section 14.4.5 may legally be made, then, at the request of Can-Fite, SKK shall surrender such Regulatory Filings and/or Marketing Authorizations for cancellation. To the extent that such assigned Regulatory Filings and/or Marketing Authorizations are related to the Product, all such data, files, materials, information, filings and approvals shall thereafter be deemed to be Can-Fite’s Confidential Information and subject to Article 13 of this Agreement. SKK further agrees to execute and deliver such instruments and take such other actions as Can-Fite shall reasonably request in order to carry out this provision.
| 40 |
Execution Copy
Confidential
14.5 Return of Confidential Information. Confidential Information shall remain the property of the Disclosing Party for the period provided in Section 13.1. Upon earlier termination of this Agreement by either Party under Section 14.2 because of uncured material breach or under Section 14.3 because of insolvency of the other Party, or by Can-Fite for SKK’s failure to proceed with Product development pursuant to Section 5.5, the Receiving Party shall immediately cease to use the Disclosing Party’s Confidential Information and promptly thereafter the Receiving Party shall, at the Receiving Party’s option, either return to the Disclosing Party or destroy all data, drawings, memoranda, notes and other written materials (including summaries, records, descriptions, modifications, drawings and adaptations that have been made from any such materials), together with any magnetic media and computer stored information, and all copies thereof, embodying or containing any of the Disclosing Party’s Confidential Information that are in the possession or control of the Receiving Party or its contractors or agents; provided, however, that one (1) copy of such Confidential Information may be retained by the Receiving Party on a confidential basis for archival purposes only. Any destruction of Confidential Information pursuant to the preceding sentence shall be promptly confirmed by a written certificate executed by an authorized officer of Receiving Party.
ARTICLE 15.
DISPUTE RESOLUTION
15.1 Negotiation. The Parties shall attempt in good faith to resolve any and all disputes that arise between them promptly, voluntarily and amicably. Any dispute arising between the Parties relating to, arising out of, or in any way connected with this Agreement, or any term or condition hereof, or the performance by either Party of its obligations hereunder (a “Dispute”), whether before or after expiration or termination of this Agreement, which is not settled by the Parties within thirty (30) days after written notice of such Dispute is first given by one Party to the other Party in writing, will be referred to a senior executive designated by Can-Fite and a senior executive designated by SKK who are authorized to settle such Dispute on behalf of their respective companies (“Senior Executives”). The Senior Executives will meet (or confer by telephone or video conference) within thirty (30) days after the end of the initial 30-day period referred to above, at a time and place mutually acceptable to both Senior Executives. If the Dispute has not been resolved by the Senior Executives within thirty (30) days after the end of the initial 30-day period referred to above (or such longer time period as may be mutually agreed upon by the Senior Executives), the Dispute will be resolved in accordance with the remainder of this Article 15.
15.2 Arbitration. If a Dispute is not resolved in accordance with Section 15.1, the Parties hereby agree to resolve such Dispute by final and binding arbitration administered under the then-current Rules of Arbitration of the International Chamber of Commerce (“ICC”).
| 41 |
Execution Copy
Confidential
15.2.1 Commencement of Arbitration Proceeding; Arbitrator. Following failure of the Senior Executives to resolve a Dispute under Section 15.1, either Party may commence such arbitration proceeding in accordance with this Section 15.2 and the ICC rules, and shall simultaneously notify the other Party in writing of such commencement. The arbitration shall be conducted by one (1) neutral arbitrator, to be mutually selected by the Parties within thirty (30) days of the commencement of the proceeding; provided that if the Parties are unable to mutually select such arbitrator within such 30-day period, then the Parties shall either mutually agree to extend such period or one neutral arbitrator will be selected by Can-Fite within such thirty (30) day period, one neutral arbitrator will be selected by SKK within such thirty (30) day period, and such two selected arbitrators shall, within thirty (30) days after the first two arbitrators have been selected, appoint the single neutral arbitrator who shall preside over the arbitration proceeding.
15.2.2 Arbitration Proceeding and Venue. The arbitration and all related hearings, proceedings and written submissions will be in the English language. The arbitration proceeding shall be held in Geneva, Switzerland (unless the Parties mutually agree in writing on a different venue). Each Party shall bear its own expenses (including the fees and expenses of its attorneys, consultants and witnesses) in connection with the arbitration proceeding, and each Party shall, on an ongoing basis, pay one-half (½) the fees and expenses of the ICC and the arbitrator(s).
15.2.3 Decision; Enforcement. The decision of the arbitrator shall be the sole and exclusive remedy of the Parties, shall be final and shall be fully and irrevocably accepted by the Parties. The arbitrator shall announce his/her decision and award, and the reasons therefor, in writing. The prevailing Party may enforce such decision against the other Party in any court having jurisdiction. In any arbitration proceeding hereunder, the arbitrator will not have the right to modify the terms and conditions of this Agreement. The Parties will exert reasonable efforts to have the decision and award rendered within six (6) months after a Party commences the arbitration proceeding.
15.3 Court Actions; Injunctive Relief. Notwithstanding the above, to the full extent allowed by law, either Party may bring an action in any court of competent jurisdiction for injunctive relief (or any other provisional remedy) to protect the Parties’ rights or enforce the Parties’ obligations under Sections 10, 13 or 16.8 of this Agreement. In addition, either Party may bring an action in any court of competent jurisdiction to resolve disputes pertaining to the validity, construction, scope, enforceability, infringement or other violations of patents or other proprietary or intellectual property rights.
| 42 |
Execution Copy
Confidential
ARTICLE 16.
MISCELLANEOUS
16.1 Force Majeure. Neither Party shall be held liable or responsible to the other Party, nor be deemed to have defaulted under or breached this Agreement, for failure or delay in fulfilling or performing any term of this Agreement when such failure or delay is caused by or results from causes beyond the reasonable control of the affected Party, including but not limited to fire, floods, earthquake, embargoes, war, acts of war (whether war is declared or not), insurrections, riots, civil commotions, strikes, lockouts or other labor disturbances, acts of God or acts, omissions or delays in acting by any governmental authority or the other Party; provided, however, that the Party so affected shall use Commercially Reasonable Efforts to avoid or remove such causes of nonperformance, and shall continue to perform hereunder with reasonable dispatch whenever such causes are removed. Either Party shall provide the other Party with prompt written notice of any delay or failure to perform that occurs by reason of force majeure. The Parties shall mutually seek a resolution of the delay or the failure to perform as noted above.
16.2 Assignment. This Agreement may not be assigned or otherwise transferred by either Party without the prior written consent of the other Party; provided that Can-Fite and SKK may assign this Agreement and all or a portion of its rights and obligations hereunder in connection with the transfer or sale of all or substantially all of the business of it, or in the event of its merger or consolidation or change in control or similar transaction upon prior written notice to the other Party. Any permitted assignee shall assume all obligations of its assignor under this Agreement in writing, and the relevant assignor shall remain liable thereunder.
16.3 Severability. If any provision hereof should be held invalid, illegal or unenforceable in any jurisdiction, the Parties shall negotiate in good faith a valid, legal and enforceable substitute provision that most nearly reflects the original intent of the Parties and all other provisions of this Agreement shall remain in full force and effect in such jurisdiction and shall be liberally construed in order to carry out the intentions of the Parties hereto as nearly as may be possible. Such invalidity, illegality or unenforceability shall not affect the validity, legality or enforceability of this Agreement in any other jurisdiction.
16.4 Notices. All notices, requests, consents and other communications given or made by a Party under this Agreement shall be in writing and shall be deemed given (i) five (5) days after mailing when mailed (by registered or certified mail, postage paid, only), (ii) on the date sent when made by facsimile transmission with confirmation of receipt (with hard copy to follow by registered or certified mail, postage paid, only), or (iii) on the date received when delivered in person or by reputable overnight courier; provided that notices and communications with respect to administrative matters under this Agreement (but not legal matters or matters pertaining to rights or obligations under this Agreement), may be provided by e-mail and will be deemed given when sent. All notices shall be provided to the address set forth below or such other place as such Party may from time to time designate in writing:
| If to Can-Fite: | Can-Fite BioPharma, Ltd. |
| 10 Bareket St. | |
| Petach Tikva, Israel | |
| Attention: Chief Executive Officer | |
| Facsimile: +972.3.924.9378 | |
| E-Mail: info@canfite.com |
| 43 |
Execution Copy
Confidential
| with a copy to: | Heller Ehrman LLP |
| 4350 La Jolla Village Drive | |
| San Diego, CA 92122 USA | |
| Attention: Stephen C. Ferruolo | |
| Facsimile: 1.858.450.8499 | |
| E-mail: Stephen.Ferruolo@hellerehrman.com | |
| If to SKK: | Seikagaku Corporation |
| 6-1, Marunouchi 1-chome | |
| Chiyoda-ku, Tokyo 100- 0005, Japan | |
| Attention: Ken Mizutani | |
| President | |
| Facsimile: 81.3.5220.8951 | |
| E-Mail: ken.mizutani@seikagaku.co.jp |
| with a copy to: | Seikagaku Corporation |
| 6-1, Marunouchi 1-chome | |
| Chiyoda-ku, Tokyo 100- 0005, Japan | |
| Attention: General Manager | |
| Intellectual Property Department | |
| Facsimile: 81.3.5220.8951 | |
| E-Mail: shunsuke.goto@seikagaku.co.jp | |
| and | |
| Seikagaku Corporation | |
| 6-1, Marunouchi 1-chome | |
| Chiyoda-ku, Tokyo 100- 0005, Japan | |
| Attention: General Manager | |
| Licensing Department | |
| Facsimile: 81.3.5220.8594 | |
| E-Mail: junichi.hosono@seikagaku.co.jp |
16.5 Governing Law, Venue. This Agreement and any dispute arising from the performance or breach hereof shall be governed by and construed and enforced in accordance with the laws of State of New York, without reference to conflicts of laws principles.
16.6 Entire Agreement; Amendment. This Agreement, together with the Exhibits hereto, contains the entire understanding of the Parties with respect to the subject matter hereof. In the event of any conflict or inconsistency between any provision of any Exhibit hereto and any provision of this Agreement, the provisions of this Agreement shall prevail. All express or implied agreements and understandings, either oral or written, heretofore made, including the Mutual Confidential Disclosure Agreement between the Parties, dated April 27, 2004, are expressly superseded by this Agreement. This Agreement may be amended, or any term hereof modified, only by a written instrument duly executed by both Parties hereto.
| 44 |
Execution Copy
Confidential
16.7 Official Language. The language of this Agreement and of any documents, papers or proceedings required by or under this Agreement, including any such documents, papers or proceedings that arise under Article 15, shall be English. Any Party requesting or requiring translations of such documents, papers or proceedings shall bear all costs and expenses of such translations.
16.8 Independent Contractors. It is expressly agreed that Can-Fite and SKK shall be independent contractors and that the relationship between the Parties shall not constitute a partnership, joint venture or agency. Neither Can-Fite nor SKK shall have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on the other, without the prior written consent of the other Party to do so.
16.9 Waiver. The waiver by either Party hereto of any right hereunder or the failure to perform or of a breach by the other Party shall not be deemed a waiver of any other right hereunder or of any other breach or failure by said other Party whether of a similar nature or otherwise.
16.10 Counterparts. This Agreement may be executed in counterparts by original or facsimile signature, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
IN WITNESS WHEREOF, the Parties hereto have caused this Agreement to be executed by their duly authorized representative as of the date first above written.
| CAN-FITE BIOPHARMA, LTD. | SEIKAGAKU CORPORATION | |||
| By: | /s/ Pnina Fishman | By: | /s/ Ken Mizutani | |
| Name: | Pnina Fishman | Name: | Ken Mizutani | |
| Title: | CEO | Title: | President | |
| By: | /s/ Ilan Cohn | |||
| Name: | Ilan Cohn | |||
| Title: | Vice Chairman | |||
| 45 |
Execution Copy
Confidential
EXHIBIT A
DESCRIPTION OF INGREDIENT

Execution Copy
Confidential
EXHIBIT B
LICENSED PATENTS
| DETAILS* | TITLE** | |
| Japanese patent application 2001-522994 | Pharmaceutical compositions comprising an adenosine receptor agonist or antagonist | |
| Japanese patent application 2003-397549 | Method of Treating an Individual with methyl 1-[N6-( 3-iodobenzyl) -adenin-9-yl]-β-D-ibofuronamide | |
| PCT application IL2005/001166 | Therapeutic Treatment of Accelerated Bone Resorption | |
| US Provisional application 60/740,631 | Treatment of osteoarthritis | |
| PCT application IL2005/001280 | Treatment of Inflammation by a Combination of Methotrexate and an A3 Adenosine Receptor Agonist |
* In case of a PCT application, the Licensed Patent is the Japanese patent that will be granted on a national Japanese patent application filed on the basis of the PCT application; in case of a US Provisional application, the Licensed Patent will be a Japanese patent which claims priority from the US Provisional application.
** The title is for identification purposes only. The title on file may be different or may be amended by Can-Fite or by the Japanese Patent Office.
Execution Copy
Confidential
EXHIBIT C
TRADEMARKS
[None Selected as of the Effective Date]
[To Be Added During the Term of the Agreement]